(UroToday.com) The 2023 ASCO annual meeting included a kidney cancer session, featuring a presentation by Dr. Thomas Hutson discussing the final prespecified overall survival analysis of CLEAR, the 4-year follow-up of lenvatinib + pembrolizumab vs sunitinib in patients with advanced RCC. In the phase 3 CLEAR trial, lenvatinib + pembrolizumab showed clinically meaningful and statistically significant benefits in progression free survival (primary endpoint) and overall survival, and improved objective response rate compared with sunitinib in first line advanced RCC.1 Based on the results from CLEAR, lenvatinib + pembrolizumab was approved by regulatory agencies for the first line treatment of adult patients with advanced RCC. At the 2023 ASCO annual meeting, Dr. Hutson and colleagues reported the final prespecified overall survival analysis of lenvatinib + pembrolizumab versus sunitinib with a median follow-up of 4 years.
Treatment-naïve patients (n=1,069) who had advanced RCC with a clear-cell component were randomized (1:1:1) to receive: lenvatinib 20 mg PO QD + pembrolizumab 200 mg IV Q3W vs lenvatinib 18 mg + everolimus 5 mg PO QD vs sunitinib 50 mg PO QD (4 weeks on/2 weeks off). Stratification factors were geographic region and MSKCC prognostic risk group. This final prespecified overall survival analysis (data cut off July 31, 2022) with 23 months additional follow-up was triggered by ~304 death events in two arms. IMDC risk group was not a stratification factor and relevant data were derived programmatically. Overall survival, progression free survival, objective response rate, duration of response, and progression free survival on next-line therapy (PFS2) were assessed for lenvatinib + pembrolizumab and sunitinib. Additionally, progression free survival, objective response rate, and duration of response were assessed per independent review using RECIST v1.1.
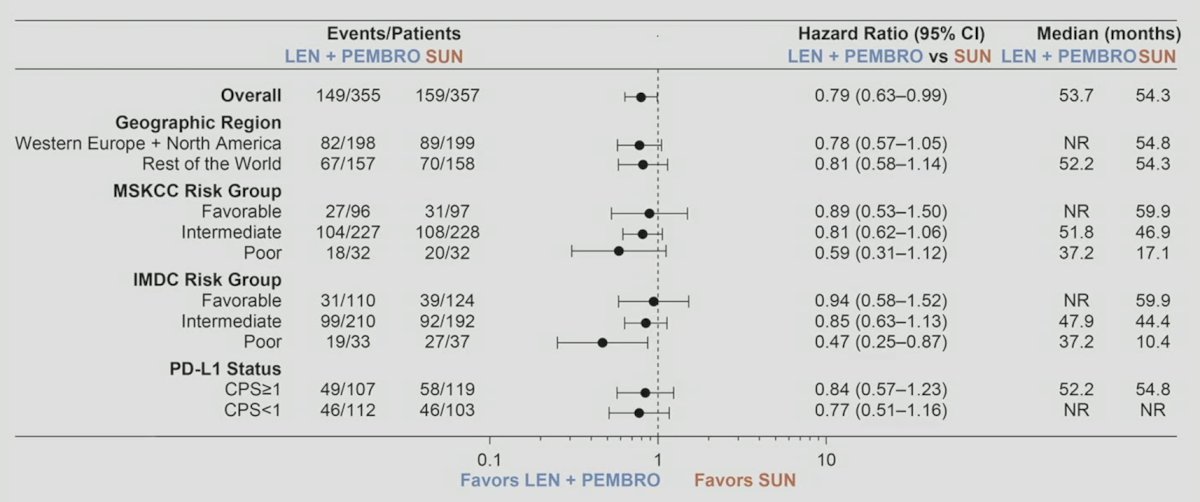
At a median follow-up of 49.8 months (IQR 41.4–53.1) for lenvatinib + pembrolizumab and 49.4 months (IQR 41.6–52.8) for sunitinib, 149 and 159 deaths had occurred, respectively. Overall survival benefit with lenvatinib + pembrolizumab vs sunitinib was maintained (HR 0.79, 95% CI 0.63–0.99):

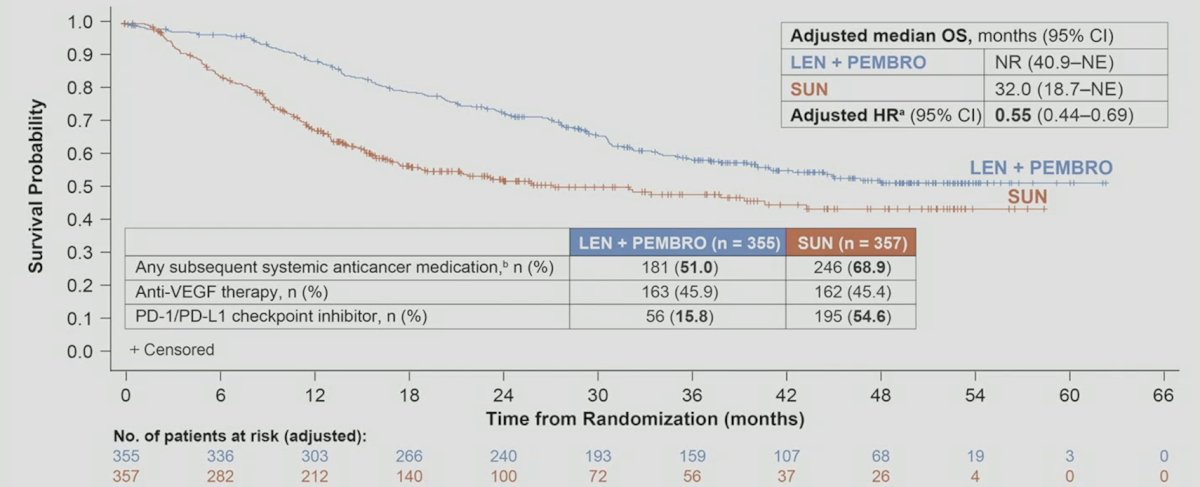
The final overall survival analysis adjusted for subsequent anticancer medicines showed a strong benefit of lenvatinib + pembrolizumab vs sunitinib: HR 0.55, 95% CI 0.44-0.69:
Furthermore, the final overall survival analysis generally showed benefits amongst subgroups of patients:
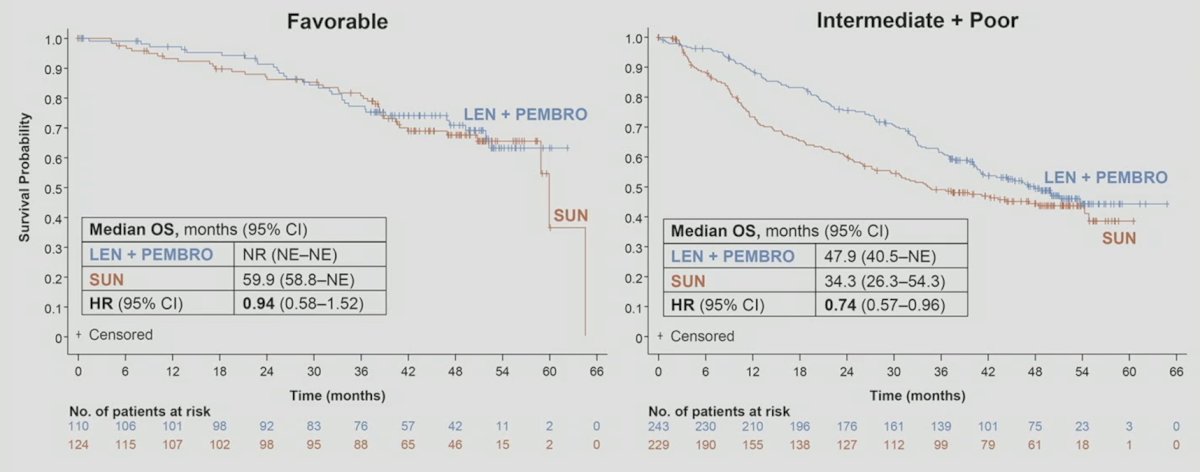
Overall survival did not favor lenvatinib + pembrolizumab vs sunitinib amongst MSKCC favorable risk patients (HR 0.94, 95% CI 0.58-1.52), but did amongst intermediate + poor risk patients: HR 0.74, 95% CI 0.57-0.96:
Also, the progression free survival benefit of lenvatinib + pembrolizumab vs sunitinib was maintained (HR 0.47, 95% CI 0.38–0.57):
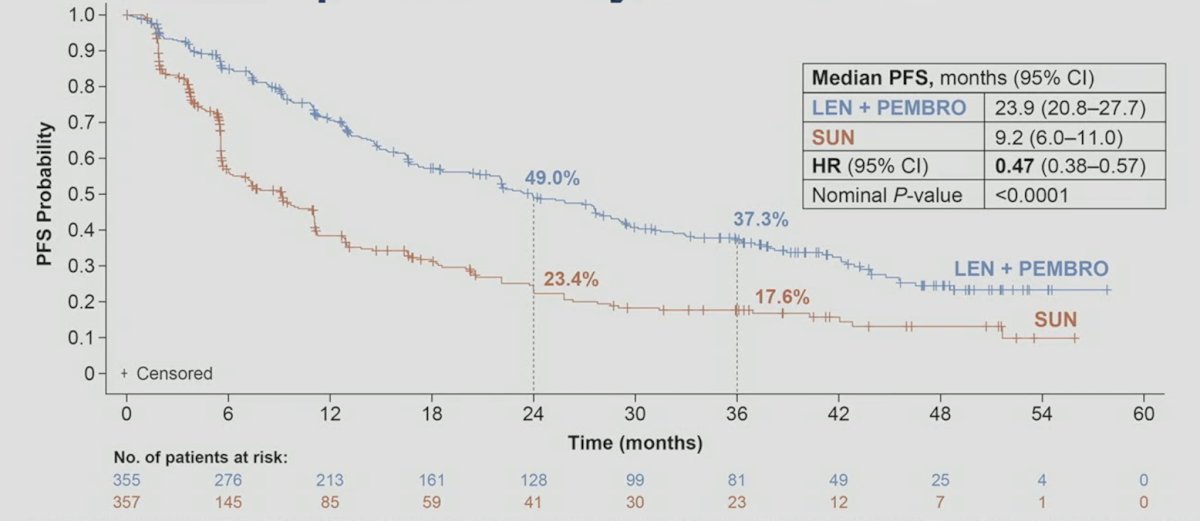
Progression free survival also favored lenvatinib + pembrolizumab vs sunitinib across MSKCC risk groups: Favorable risk – HR 0.50, 95% CI 0.35, 0.35–0.71 and intermediate + poor risk - HR 0.43, 95% CI 0.34–0.55. Furthermore, objective response rate was greater with lenvatinib + pembrolizumab (71.3%; complete response, 18.3%) vs sunitinib (36.7%; complete response, 4.8%) (relative risk 1.94, 95% CI 1.67–2.26). Duration of response significantly favored lenvatinib + pembrolizumab vs sunitinib, with a HR of 0.57, 95% CI 0.43-0.76:

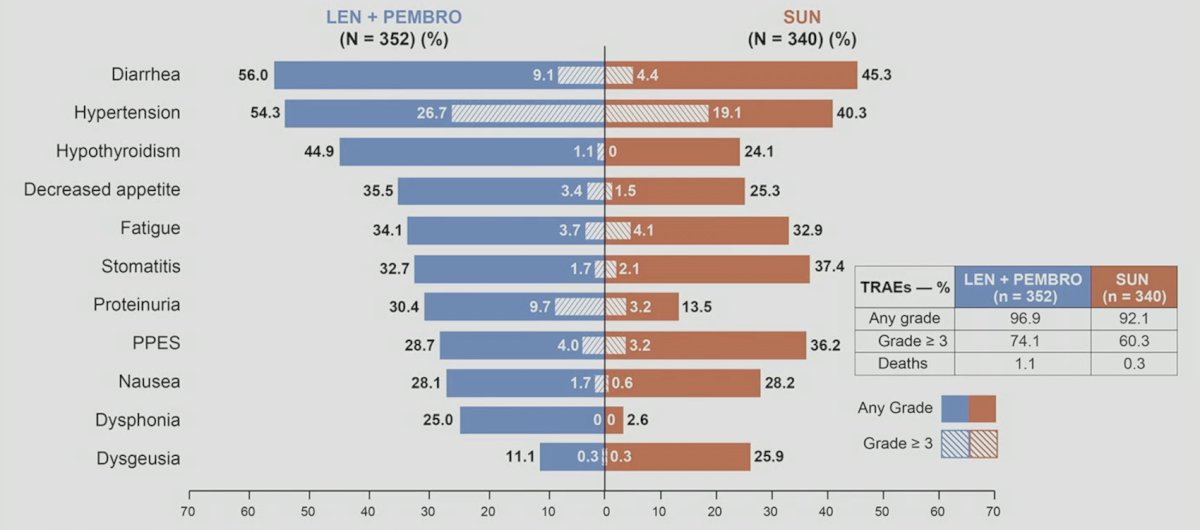
Less patients in the lenvatinib + pembrolizumab arm (181/355, 51.0%) received subsequent anticancer therapies compared with the sunitinib arm (246/357, 68.9%), including 56 (15.8%) and 195 (54.6%) that received PD-1/PD-L1 checkpoint inhibitors, respectively. PFS2 was longer with lenvatinib + pembrolizumab vs sunitinib (43.3 vs 25.9 months; HR 0.63, 95% CI 0.51–0.77). Grade ≥3 treatment-related adverse events occurred in 74.1% and 60.3% patients in the lenvatinib + pembrolizumab and sunitinib arms, respectively. The treatment related adverse events with >= 25% frequency is as follows:
Dr. Hutson concluded his presentation discussing the final prespecified overall survival analysis of CLEAR with the following take-home points:
- Lenvatinib + pembrolizumab continues to demonstrate clinically meaningful benefit vs sunitinib in overall survival, progression free survival, objective response rate, and complete response in the first line treatment of patients with advanced RCC at 4-year follow-up
- There were no new safety signals and adverse events were managed with dose modifications as necessary
- These results support the robustness of the primary analysis data from CLEAR
Presented by: Thomas E. Hutson, DO, Texas Oncology, Dallas, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021 Apr 8;384(14):1289-1300.