(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to an oligometastatic renal cell carcinoma (RCC) session. Dr. Karen Runcie discussed the role of systemic therapy for the treatment of such patients.
Dr. Runcie began with a case presentation of a 64-year-old female with an incidentally found 8 cm renal mass. On staging work-up with a CT scan, the patient was found to have a 2 cm rib lesion, that was biopsy-confirmed clear cell RCC. The patient was also found to have a single pulmonary metastatic lesion in the left lung lower lobe. By the International Metastatic RCC Database Consortium (IMDC) risk criteria, the patient was deemed IMDC intermediate risk (1 risk factor). Dr. Runcie proceeded to present supporting evidence for systemic therapy in the oligometastatic setting.
The first question to answer in this setting is: Do we need to treat this patient? The current NCCN guidelines suggest that active surveillance is a reasonable management approach for select patients with favorable risk metastatic RCC.
What is the evidence to support this recommendation? In a prospective phase II trial of 52 patients with treatment-naive, asymptomatic, metastatic RCC, Rini et al. demonstrated that active surveillance for select metastatic RCC patients is feasible with a median delay to systemic therapy of 14.9 months. On multivariable analysis, patients with higher numbers of IMDC adverse risk factors (p=0.04) and higher numbers of metastatic disease sites (p=0.04) had significantly shorter surveillance periods. This is further demonstrated in the Kaplan Meier curve below whereby patients with favorable risk features had a median delay to systemic therapy of 22.2 months, compared to 8.4 months in patients with unfavorable risk features.1 Additional analyses have also demonstrated that patients with TP53 and SMARC4 mutations progress faster than those without these mutations.
What about the role of cytoreductive nephrectomy in this setting? The early evidence for cytoreductive nephrectomy emerged in the era of interferon-alpha treatment for patients with metastatic RCC. The EORTC 309472 and SWOG 89493 trials both demonstrated that cytoreductive nephrectomy was associated with overall survival benefits, as demonstrated in the Kaplan Meier curves below:
The CARMENA trial evaluated the role of cytoreductive nephrectomy in the sunitinib era. This trial randomized patients with metastatic clear cell RCC to upfront cytoreductive nephrectomy followed by sunitinib versus upfront sunitinib followed by potential nephrectomy, as per investigator discretion (18% of patients in this arm). The results in the upfront sunitinib arm were non-inferior to those in the upfront cytoreductive nephrectomy arm with regards to overall survival (HR: 0.89; 95% CI: 0.71 - 1.10; upper boundary of the 95% confidence interval for noninferiority, ≤1.20), with median overall survivals of 18.4 months and 13.9 months, respectively.5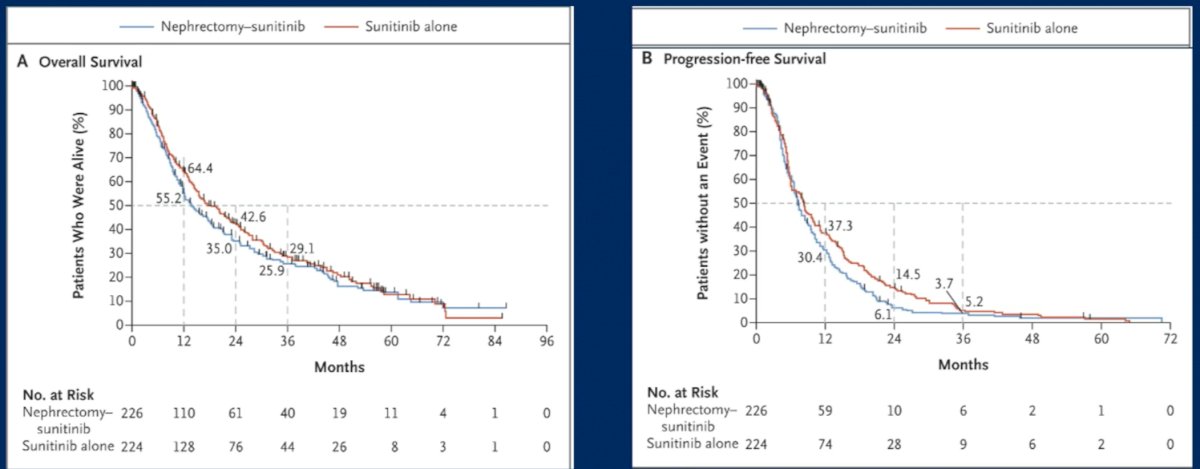
The SURTIME trial, which closed prematurely due to poor accrual, similarly demonstrated that there was no benefit to upfront cytoreductive nephrectomy. In this trial, 99 patients were randomized to immediate cytoreductive nephrectomy followed by sunitinib therapy versus treatment with three cycles of sunitinib followed by cytoreductive nephrectomy in the absence of progression followed by sunitinib therapy. Patients in the deferred cytoreductive nephrectomy arm had significantly better overall survival outcomes with a median survival of 32.4 months versus 15.0 months in the immediate cytoreductive nephrectomy arm (HR: 0.57, 95% CI: 0.34 – 0.95, p=0.03).5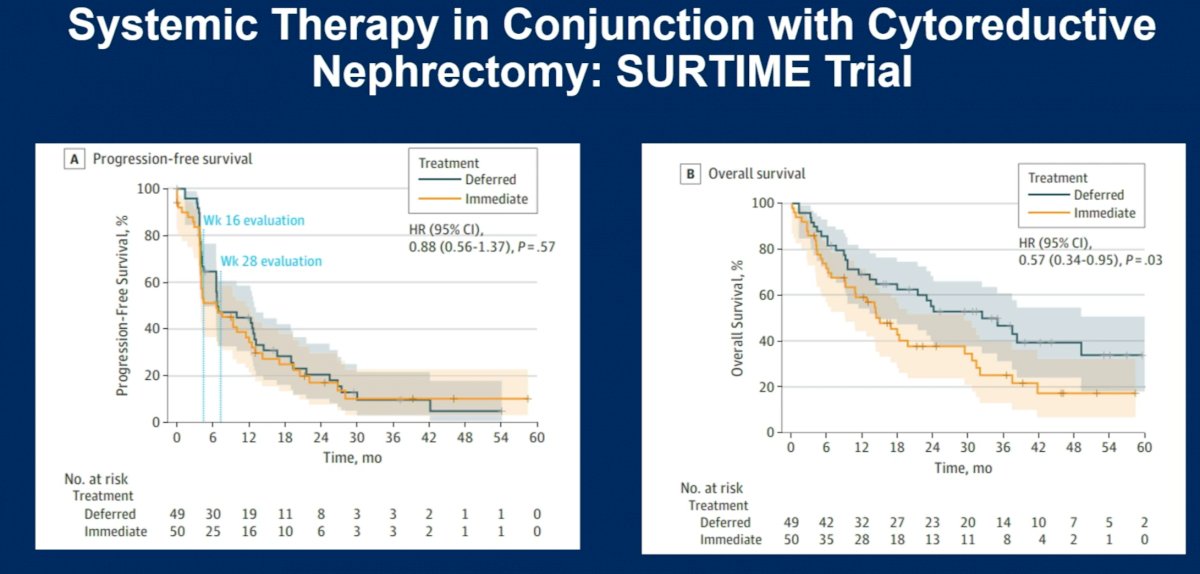
There are currently numerous ongoing studies evaluating cytoreductive nephrectomy in the immune checkpoint inhibitors combination therapy era.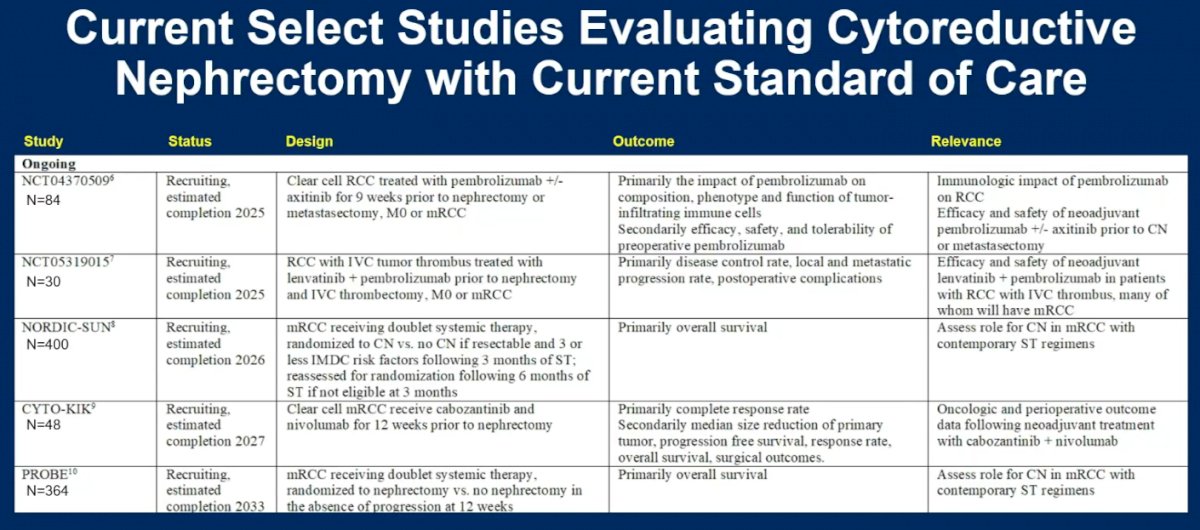
We can also use trial data from studies evaluating adjuvant therapy in the post-nephrectomy setting to inform clinical decision-making for the treatment of patients with oligometastatic disease. M1 NED patients, meaning those without evidence of residual disease following a nephrectomy plus a metastasectomy, performed up to one year following nephrectomy, appeared to derive the greatest disease-free survival benefit following one year of adjuvant pembrolizumab in the KEYNOTE-564 trial (HR: 0.29, 95% CI: 0. 12 – 0.69).6 Overall survival data is pending. Pembrolizumab is currently only agent FDA approved in the adjuvant setting for patients with clear cell RCC considered M1 NED. Adjuvant pembrolizumab did not adversely affect quality of life. About 20% of patients had a serious adverse event or discontinued pembrolizumab because of any adverse event.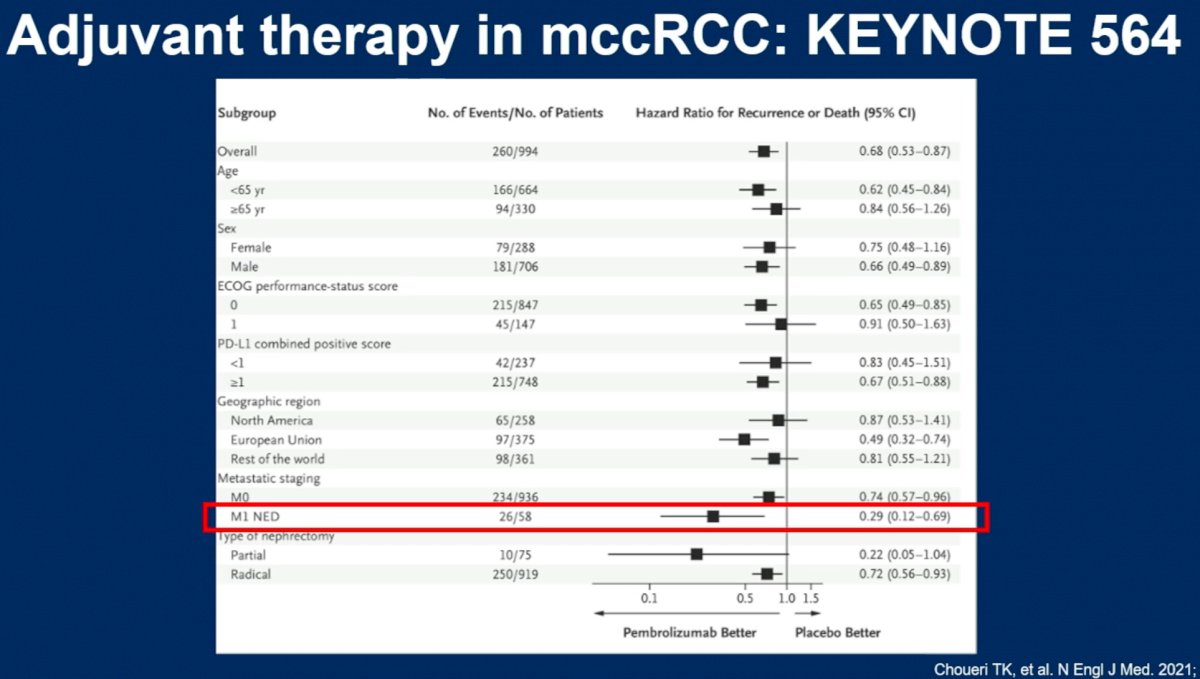
Dr. Runcie noted that in patients who are not candidates for cytoreductive nephrectomy and for whom active surveillance is not appropriate, systemic therapy with immune checkpoint inhibitor combination therapy remains the current standard of care. Currently approved agents in this setting are summarized in the illustration below.
Irrespective of the treatment chosen, Dr. Runcie emphasized that studies have shown that most cancer patients favor shared decision-making with their physician, which requires a comprehensive understanding of patient preferences. Small discrete choice experiments show that patients with advanced RCC value health-related quality of life and overall survival the most, followed by serious adverse events when making treatment decisions.
Dr. Runcie concluded her presentation with the following take home messages:
- There may be a role for active surveillance in patients with low tumor burden and favorable risk disease
- For patients with metastatic clear cell RCC who undergo nephrectomy and metastasectomy within one year of nephrectomy, adjuvant pembrolizumab has been shown to significantly prolong disease-free survival.
- Patients with unfavorable IMDC risk and those with hepatic, brain, or bone metastases should consider upfront systemic therapy instead of metastasis-directed therapy.
Presented by: Karie Runcie, MD, Assistant Professor, Division of Hematology/Oncology, Department of Internal Medicine, Columbia University Irving Medical Center, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:- Rini BI, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol, 2016;17(9):1317-24
- Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet, 2001;358(9286):966-70.
- Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer. N Engl J Med 2001;345:1655-9.
- Mejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med, 2018 2;379(5):417-427.
- Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol, 2019;5(2):164-170.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021;385(8):683-94.


