(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting included a prostate cancer session, featuring a trials-in-progress presentation by Dr. Neal Shore discussing ARASEC, an open-label study of darolutamide plus ADT vs ADT in metastatic hormone-sensitive prostate cancer (mHSPC) using an external control arm. Darolutamide is a structurally distinct and highly potent androgen receptor inhibitor. In the phase 3 ARASENS study (NCT02799602) in patients with mHSPC, darolutamide + ADT + docetaxel significantly reduced the risk of death by 32.5% vs ADT + docetaxel (HR 0.68; 95% CI 0.57–0.80), with a favorable tolerability and safety profile.1 In the global (excluding US), phase 3 ARANOTE study (NCT04736199), darolutamide + ADT vs ADT alone is being evaluated in patients with mHSPC. ARASEC (NCT05059236) will complement ARANOTE by assessing darolutamide + ADT in US patients. As ADT alone is no longer an acceptable comparator in the US, the external control arm will be derived from the historical US CHAARTED study (NCT00309985).2 A real-world analysis of electronic health record data from patients with mHSPC (TIMES) indicated that there was minimal risk of historical time bias associated with the use of the external ADT alone arm from CHAARTED as a comparator for ARASEC.3
ARASEC is a phase 2, open-label, single-arm study enrolling patients with confirmed mHSPC and no prior systemic therapy. Patients will receive darolutamide 600 mg orally twice daily + ADT (luteinizing hormone-releasing hormone agonist/antagonist or orchiectomy). The external control arm will be derived from 394 patients with mHSPC treated with ADT alone in CHAARTED. To remove biases in evaluating outcomes that could arise due to differences in baseline profiles between arms, patients will be matched 1:1 using a propensity score summarizing baseline characteristics relevant to disease outcomes. The trial design for ARASEC is as follows:
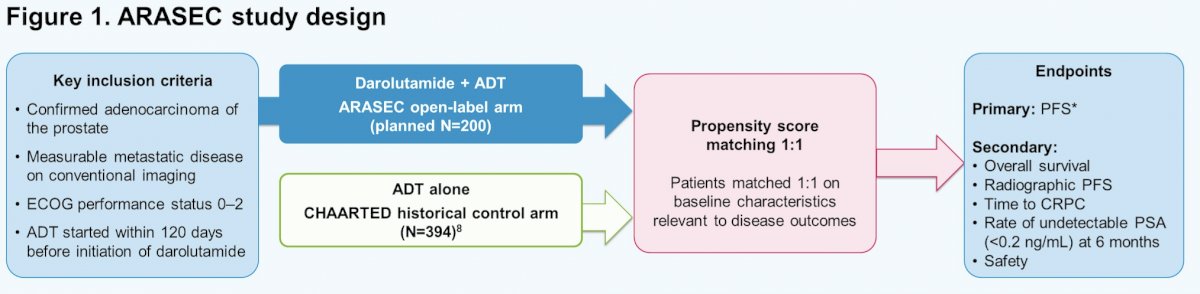
Baseline variables will include age (< 70 vs ≥70 years), Eastern Cooperative Oncology Group performance status (0–1 vs 2), CHAARTED-defined extent of disease (low vs high volume + bone metastases only vs high volume + visceral metastases ± bone metastases), and prior therapy (yes vs no; as a proxy for recurrent vs de novo disease). Matching patients with scores within a narrow window across the cohorts will ensure that patients in CHAARTED are representative of patients in the darolutamide + ADT arm. The primary endpoint is progression-free survival (PFS), as defined in CHAARTED. Secondary endpoints include:
- Overall survival
- Radiographic PFS
- Time to CRPC
- Undetectable PSA response rate (< 0.2 ng/mL) at 6 months
- Safety
As of May 2023, ARASEC enrollment is complete, with 222 patients enrolled from 29 sites:
The primary endpoint analysis will take place after 161 PFS events have occurred and primary completion is anticipated in June 2024.
Presented by: Neal Shore, MD, FACS, U.S. Chief Medical Officer of Surgery and Oncology, GenesisCare USA, Director, CPI, Carolina Urologic Research Center, Carolina Urologic Research Center, Myrtle Beach, SC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
- Shore N, et al. Open-label study of darolutamide plus androgen-deprivation therapy (ADT) vs ADT in metastatic hormone-sensitive prostate cancer using an external control arm (ARASEC). J Clin Oncol 2022;40(28 Suppl): Abstract 403


