(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Tian Zhang discussing the primary analysis of STARTAR, a phase 2 salvage trial of androgen receptor inhibition with ADT and apalutamide with radiation therapy followed by docetaxel in men with PSA recurrent prostate cancer after radical prostatectomy. ADT with salvage radiotherapy improves survival for men with PSA recurrence after radical prostatectomy. For high risk PSA recurrence, the current standard ADT duration is up to 2 years with radiotherapy, however shortening but intensifying systemic therapy may improve outcomes. Previously, the STREAM trial showed that 6 months enzalutamide with ADT/radiotherapy had 3-year progression free survival of 53% in a high risk population, including lymph node positive patients.1 Given docetaxel improves survival in mHSPC, Dr. Zhang and colleagues evaluated the combination of salvage radiotherapy, ADT/apalutamide, and docetaxel in this setting.
STARTAR is a multicenter investigator initiated phase 2 trial for salvage treatment of PSA recurrent prostate cancer post-radical prostatectomy, conducted in the Department of Defense Prostate Cancer Clinical Trials Consortium. Key inclusion criteria included:
- Gleason 7 with T3/positive margin/N1 or Gleason 8-10 prostate cancer
- PSA relapse <4 years post-radical prostatectomy (inclusion PSA 0.2-4 ng/mL)
- and <4 positive lymph nodes, with negative standard imaging
Patients received ADT with apalutamide for 9 months, radiotherapy (66-74 Gy to the prostate bed +/- pelvic lymph nodes over 6-8 weeks) starting week 8, and then completed 6 cycles of concurrent docetaxel 75mg/m2 q3 weeks. The trial design for STARTAR is as follows:

The primary endpoint was 36 month progression free survival, defined as composite of freedom from PSA > 0.2 ng/mL + post-radiotherapy nadir with subsequent rise, clinical progression, start of other therapy, or death, among patients with testosterone recovery (>100 ng/dL). Kaplan-Meier estimates for progression free survival were used to determine landmark 24 month and 36 month rates and were compared to progression free survival rates from prior trials using a binomial test.
From March 2018 to February 2020, 39 patients were enrolled. As of the December 2022 data cutoff, the median follow up was 36 months. Baseline characteristics included Gleason 7 in 54% of patients, Gleason 8-10 in 46%, 23% lymph node positive, median PSA 0.58 ng/mL (range 0.21-3.40), and median time from radical prostatectomy of 7.6 months (range 2-98). All patients completed study treatment (ADT, apalutamide, radiation, and docetaxel) and there were 37 patients (95%) and 23 patients (62%) that completed at least 1 and all 6 cycles docetaxel, respectively. All patients achieved undetectable PSA nadirs. At 24 months and 36 months, progression free survival rates were 84% and 72%, respectively, with 95% patients recovering testosterone at 36 months:
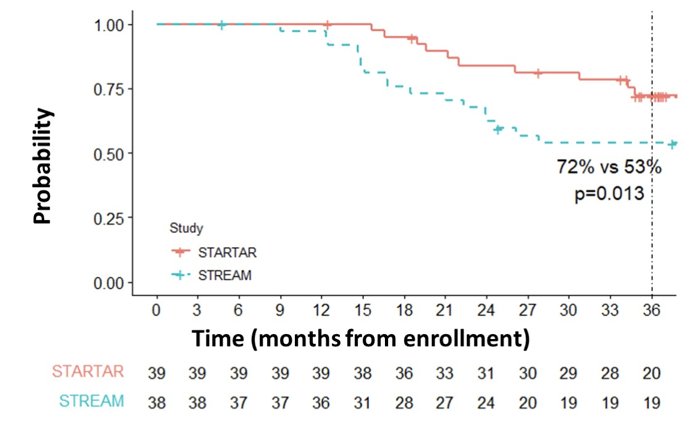
Compared to 40% historical progression free survival and 53% STREAM progression free survival rates, the 72% 36-month progression free survival rate was statistically significantly improved (p<0.001 and p=0.013, respectively). There were 38 patients (97%) that have recovered their testosterone level to the normal range:

Common any-grade adverse events included 98% hot flashes, 88% fatigue, 77% alopecia, 57% dysgeusia, and 53% rash (28% grade 1; 15% grade 2, 10% grade 3), with 5% febrile neutropenia:

Hematologic adverse events included 27 (70%) grade 3-4 neutropenia and 23 (59%) grade 1-2 anemia.
Dr. Zhang concluded her presentation discussing the primary analysis of STARTAR with the following take-home points:
- In this first phase 2 trial of ADT, apalutamide, salvage radiotherapy, and 6 cycles of docetaxel for high risk PSA recurrence, the primary endpoint 3-year progression free survival rate improved to 72%, indicating durable remissions beyond historic controls
- All but one patient recovered testosterone by 3 years (median time 15 months), with the majority of patients remaining PSA progression free with full testosterone recovery
- Intensifying systemic treatments in the non-metastatic hormone sensitive but high risk salvage setting may be feasible and efficacious, with fewer patients experiencing cancer progression over time
Presented by: Tian Zhang, MD, University of Texas Southwestern Medical Center, Dallas, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:


