(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Daniel George discussing a prospective trial of apalutamide and abiraterone acetate plus prednisone in black and white men with metastatic castrate-resistant prostate cancer (mCRPC). Abi Race was the first prospective parallel cohort study of abiraterone acetate + prednisone in Black and White men with mCRPC and demonstrated greater PSA response, time to PSA progression, and toxicity rates among Black patients.1 Furthermore, ACIS (abiraterone, apalutamide)2 and Alliance A0311201 (abiraterone, enzalutamide)3 demonstrated greater radiographic progression free survival for combination AR inhibitor + abiraterone versus monotherapy but no OS benefit in mostly white mCRPC populations. Dr. George and colleagues designed the PANTHER trial to estimate clinical outcomes among Black and White patients with mCRPC treated with apalutamide, abiraterone, plus prednisone.
This parallel cohort multicenter study treated androgen receptor pathway inhibitor naïve mCRPC patients with oral apalutamide (240 mg/d), abiraterone (1000 mg/d), and prednisone (10 mg/d) continuously until disease progression, unacceptable toxicity or 2 years. The primary endpoint was radiographic progression free survival, whereas secondary endpoints were to estimate time to PSA progression, overall survival, and best PSA response, among Black and White patients, separately. Exploratory endpoints included safety and correlative biomarkers of outcome by race and ancestry.
Between July 2017 and January 2021, this study enrolled 43 Black and 50 White patients from eight sites. Baseline prognostic characteristics were largely similar with some differences:
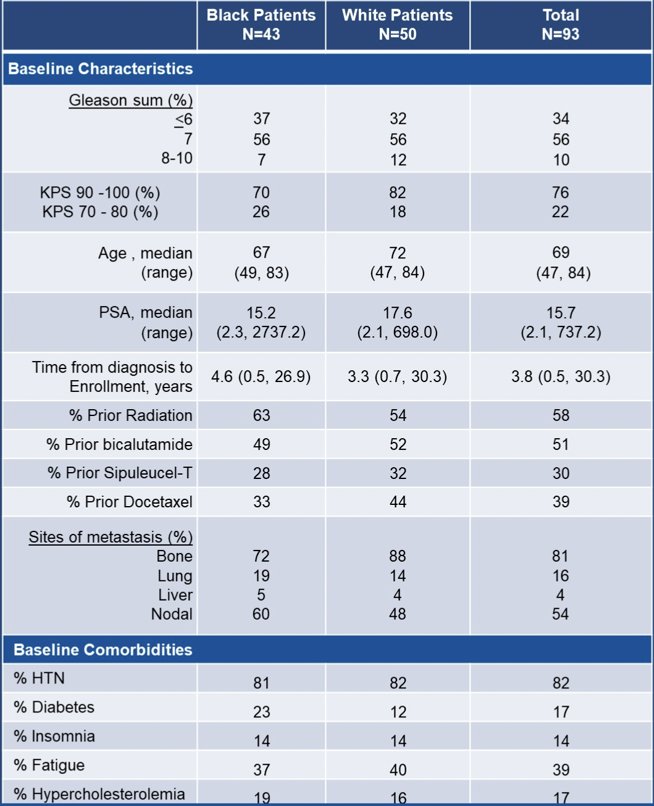
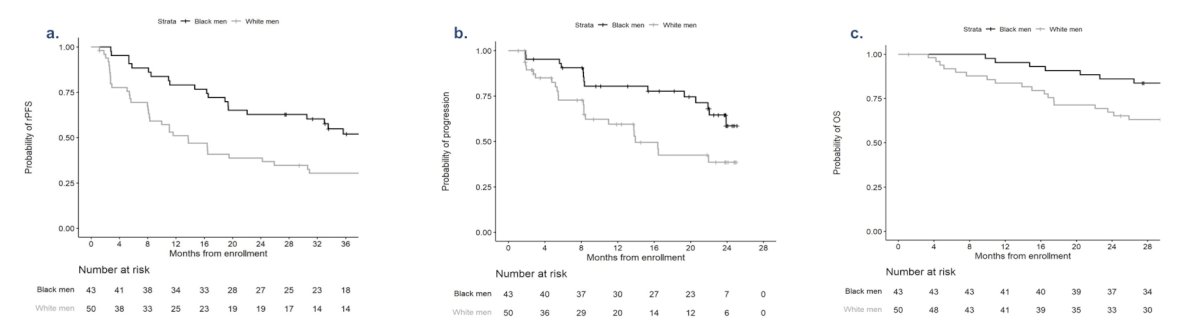
At ASCO 2023, Dr. George and colleagues reported an interim analysis as the patients are still followed for overall survival. At the time of the data submission, there were 20 and 37 radiographic progression free survival events and 16 and 32 deaths in Black and White patients, respectively. Median follow-up for Black patients was 44 months and 54 months for White patients. The Black cohort in this study had greater rPFS, time to PSA progression, and overall survival than White patients:
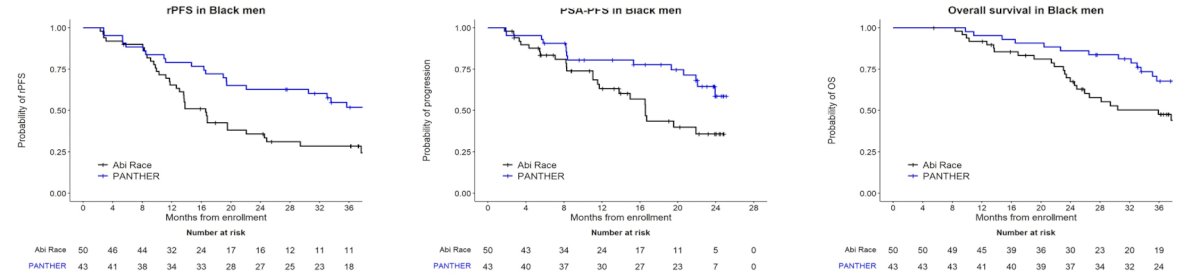
Compared to the previous study of abiraterone in mCRPC patients by Black and White cohorts (Abi Race), the Black cohort from PANTHER (abiraterone + apalutamide) demonstrated greater radiographic progression free survival, time to PSA progression, and overall survival:
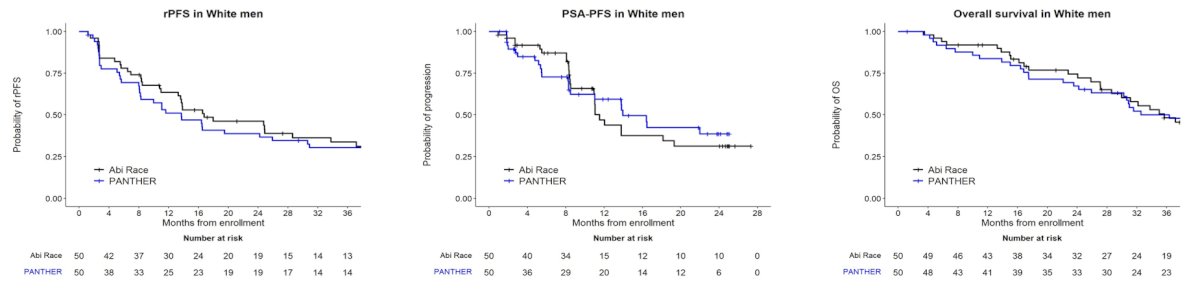
while the White cohort in PANTHER revealed similar outcomes to the Abi Race White cohort:
The combination of abiraterone and apalutamide demonstrated acceptable toxicity rates in both cohorts, with some differences by race:

Importantly, there was a higher rate of hyperglycemia (21% vs 2%), hypokalemia (51% vs 30%), and pain in extremities (30% vs 20%) in Black patients and higher rates of fatigue (54% vs 44%) in white men, similar to the Abi Race differences.
Dr. George concluded his presentation by discussing PANTHER, a prospective trial of apalutamide and abiraterone acetate plus prednisone in black and white men with mCRPC with the following take-home points:
- This study hypothesized that treatment with the combination of apalutamide + abiraterone + prednisone may result in clinical efficacy in Black men with mCRPC
- Black patients were underrepresented in the phase III ACIS trial
- Further studies of apalutamide + abiraterone + prednisone combination therapy among Black men with advanced prostate cancer are needed to determine potential clinical benefits in this understudied population
- In general, possible differences in outcomes by race can only be found if adequate numbers of Black and White patients are studied in prospective trials. More trials dedicated to addressing populations of disproportionately affected, but underrepresented, patients should be done. If not, we could miss a survival benefit for Black patients
Presented by: Daniel J. George, MD, Duke Cancer Institute Center for Prostate and Urologic Cancers, Durham, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- George DJ, Halabi S, Heath EI, et al. A prospective trial of abiraterone acetate plus prednisone in Black and White men with metastatic castrate-resistant prostate cancer. Cancer. 2021 Aug 15;127(160:2954-2965.
- Saad F, Efstathiou, Attard G, et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): A randomized, placebo-controlled, double-blind, multinational, phase 3 study. Lance Oncol. 2021 Nov;22(11):1541-1559.
- Morris MJ, Heller G, Hillman DW, et al. Randomized Phase III study of Enzalutamide compared with Enzalutamide plus abiraterone for metastatic castration-resistant prostate cancer (Alliance A031201 Trial). J Clin Oncol. 2023 Mar 30 [Epub ahead of print].


