(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a trials in progress presentation by Dr. Mohammad Atiq discussing a study of prospective monitoring of patients with recurrent prostate cancer using serial 18F-DCFPyL PSMA imaging. 18F-DCFPyL PSMA PET imaging is now FDA approved for patients with high-risk prostate cancer (based on data from the OSPREY trial1) and those with biochemically recurrent prostate cancer (based on data from the CONDOR trial2). There are an estimated 25,000-50,000 new cases of biochemically recurrent prostate cancer in the US annually. Biochemically recurrent prostate cancer has been traditionally defined as a rising PSA after definitive surgery and/or radiation have not been curative in patients without tumor findings on a CT or Tc99 bone scan. These patients are typically managed conservatively, often with surveillance since there is no data demonstrating interventions improve survival. Although 18F-DCFPyL will be able to detect disease at the micrometastatic/subclinical level, there are limited prospective data on how these patients should be managed. While great enthusiasm exists to utilize radiation or ADT-based regimens extrapolated from a more advanced disease state, the natural history of subclinical disease seen on 18F-DCFPyL remains unclear.3
The primary objective of this study is to monitor patients with recurrent prostate cancer to learn about the natural history of 18F-DCFPyL PET positive disease in this patient population. Eligible patients include:
- Those who have had prior definitive surgery or radiation
- Current PSA ≥0.50 ng/mL
- Testosterone > 100 ng/mL
- No evidence of soft tissue disease on CT scan/MRI
- No bone lesions on bone scan
Key exclusion criteria are as follows:
- MRI as clinically indicated (lymph nodes up to 1.5 cm in the shortest dimension are allowed)
- Evidence of bone lesion on Tc99 bone scan
- Prostatectomy within 1 year before entering the study
- ADT within 6 months before entering the study
- Systemic therapy for prostate cancer within 6 months before entering the study
Patients will undergo initial imaging with 18F-DCFPyL scans, and those with negative findings on the initial scan will have annual repeat 18F-DCFPyL scans. Patients with positive initial scans will be scanned every 6 months, all patients will have PSA testing every 3 months and will undergo annual CT and Tc99 bone scans. The trial design is as follows:
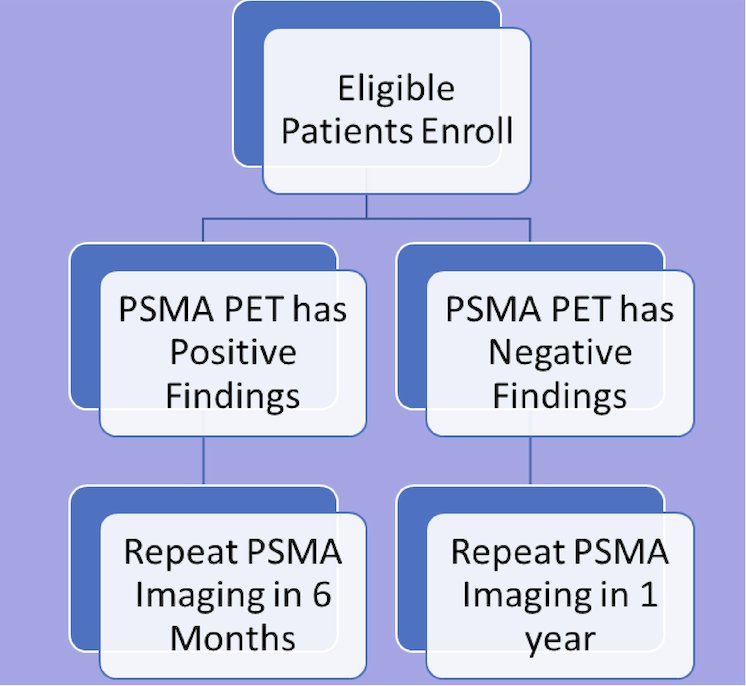
Up to 250 patients will be followed for 5 years or until off-study criteria are met (including new findings on CT/bone scans consistent with metastatic disease or continuous systemic prostate cancer therapy for more than 6 months). In addition to monitoring the imaging changes, Dr. Atiq and colleagues will evaluate patterns of progression on 18F-DCFPyL scans over time, analyze PSA doubling time in context of 18F-DCFPyL changes, and evaluate circulating biomarkers.
Presented by: Mohammad O. Atiq, MD, Genitourinary Malignancies Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021 Jul;206(1):52-61.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.
- Madan RA, Mena E, Lindenberg L, et al. With New Technology Comes Great Responsibility: Prostate-Specific Membrane Antigen Imaging in Recurrent Prostate Cancer. J Clin Oncol. 2022 Sep 10;40(26):3015-3019.


