(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers poster discussion session. Dr. Daniel Sentana Lledo presented an updated analysis of CHAARTED evaluating patient-reported quality of life (QoL) and survival outcomes in metastatic hormone-sensitive prostate cancer (mHSPC) patients.
The CHAARTED trial has previously demonstrated that the addition of docetaxel to ADT significantly improves overall survival (OS) in patients with mHSPC (HR: 0.61, 95% CI: 0.47 to 0.80).1 Furthermore, the addition of docetaxel in this setting is associated with QoL improvements at 12 months. However, to date, the prognostic relationship of QoL for OS outcomes, adjusted for disease/patient characteristics, has not yet been evaluated in this cohort.
In this study, patients were randomized in a 1:1 fashion to ADT plus docetaxel (6 cycles of 75 mg/m2 every 3 weeks) or ADT alone. QoL was evaluated using the Functional Assessment of Cancer Therapy – Prostate (FACT-P) at 3-month intervals. The association between QoL and OS was assessed using Kaplan Meier curves, with comparisons performed using the log-rank test, and Cox proportional hazards modeling, adjusted for disease/patient characteristics.
This analysis included a total of 790 patients, with 397 and 393 in the intervention and control arms, respectively.
On univariable analysis, higher baseline QoL scores were associated with improved OS outcomes (HR:0.70, 95% CI: 0.55-0.90, p=0.005); however, this association was no longer significant after adjusting for baseline patient/disease characteristics on multivariable analysis (HR: 0.80, 95% CI: 0.62 - 1.04, p=0.09).
Among patients in the lowest baseline QoL quartile, there was a trend towards improved OS in patients treated with ADT + docetaxel (HR: 0.75, 95% CI: 0.53 – 1.05, p=0.09). In contrast, patients in the highest baseline QoL quartile had similar OS outcomes irrespective of treatment arm (HR: 0.92, 95% CI: 0.63 - 1.36, p=0.69).

Significantly, a higher 3-month FACT-P score was associated with improved OS on multivariable modeling (HR: 0.76, 95% CI: 0.58 – 1.00, p=0.05). Among patients within the highest 3-month QoL group, survival outcomes did not differ by treatment arm (HR: 1.11, 95% CI: 0.73 – 1.67, p=0.63). Conversely, patients in the lowest 3-month QoL group had improved OS outcomes with ADT + docetaxel (HR: 0.69, 95% CI: 0.48 – 0.99, p=0.047).
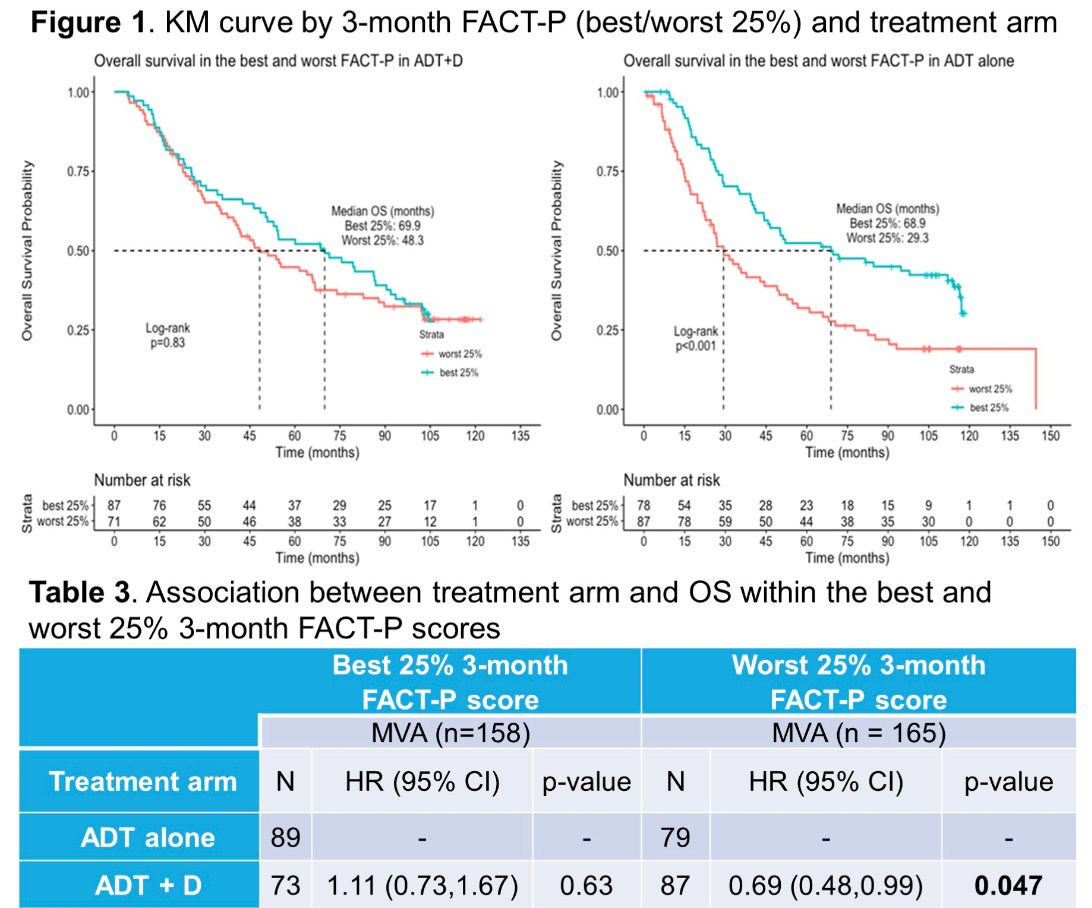
Dr. Lledo and colleagues concluded that FACT-P assessed 3-month QoL score was prognostic of OS outcomes in mHSPC patients from the CHAARTED trial. Patients with the lowest QoL (i.e., "most symptomatic”) appeared to experience improved OS outcomes with combination ADT + docetaxel, independent of disease volume. Conversely, patients with the highest 3-month QoL scores did not experience further OS benefits with addition of docetaxel, despite a predominance of patients with high-volume disease in that cohort (63%). These findings suggest that consideration of QoL measures may enhance decision-making and patient selection when considering chemohormonal treatment for mHSPC.
Presented by: Daniel Sentana Lledo, MD, Clinical Fellow, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:


