(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Shanshan Wang discussing a subpopulation analysis of the phase 3 ARASENS study assessing the efficacy and safety of darolutamide in Chinese patients with metastatic hormone-sensitive prostate cancer (mHSPC). Darolutamide is an androgen receptor inhibitor approved in China for the treatment of nonmetastatic castration-resistant prostate cancer. In the global ARASENS (NCT02799602) phase trial, darolutamide + ADT + docetaxel significantly reduced risk of death by 32.5% (HR 0.68, 95% CI 0.57–0.80) in patients with mHSPC.1 At the 2023 ASCO annual meerting, Dr. Wang and colleagues presented the efficacy and safety of darolutamide in the Chinese subpopulation of ARASENS.
In this randomized, double-blind phase 3 trial, 1,305 patients with mHSPC were randomized 1:1 to receive darolutamide 600 mg orally twice daily or placebo, in combination with ADT and docetaxel. The primary endpoint was OS and the trial design is as follows:
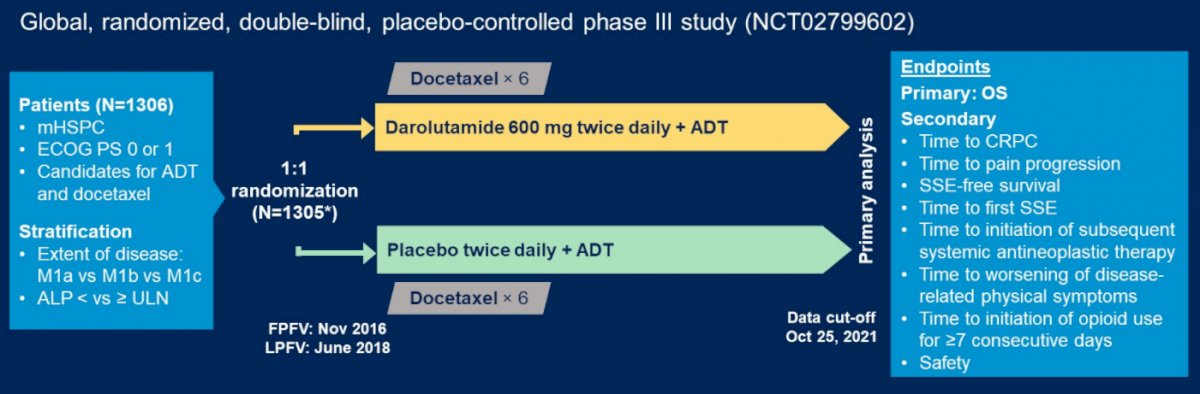
In mainland China, 202 patients were randomized to the darolutamide group (n = 104) or the placebo group (n = 98). Demographics and baseline characteristics were well balanced between treatment groups and comparable to the overall ARASENS population, except for a slightly higher proportion of patients with Gleason score ≥8, higher proportion with metastatic disease at diagnosis, and higher median baseline PSA in Chinese patients compared to the overall population. Similar to the overall intention to treat analysis, the risk of death was reduced by 36% (HR 0.64, 95% CI 0.41–0.99) with darolutamide vs placebo in Chinese patients: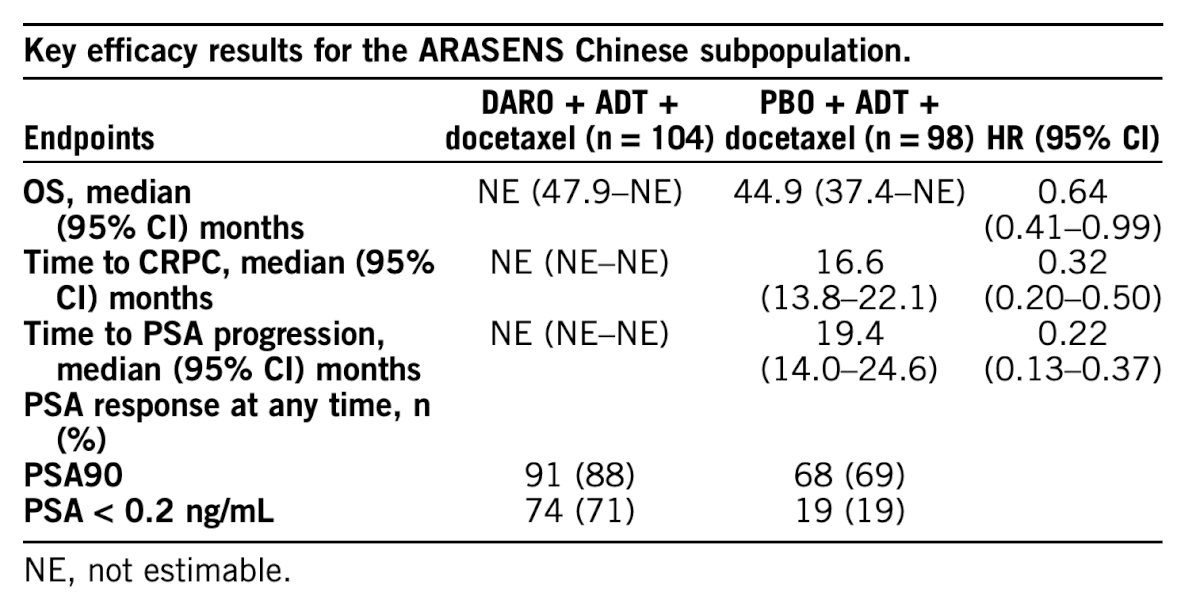
The key secondary endpoint time to castration-resistant prostate cancer (HR 0.32, 95% CI 0.20–0.50) and the exploratory endpoint time to PSA progression (HR 0.22, 95% CI 0.13–0.37) also favored darolutamide. In the darolutamide group, 81% of patients had a maximum PSA decline of ≥90% from baseline at week 12, while 88% had a maximum PSA decline of ≥90%, and 71% reached PSA < 0.2 ng/mL at any time. Incidences of treatment-emergent adverse events in Chinese patients were similar between treatment groups and comparable to the overall population. The most common grade 3/4 treatment-emergent adverse events in Chinese patients was neutropenia (darolutamide 64%, placebo 62%); grade 3/4 febrile neutropenia occurred in 4.0% of Chinese patients compared with 7.6% in the overall population.
Dr. Wang concluded this presentation discussing a subpopulation analysis of ARASENS assessing the efficacy and safety of darolutamide in Chinese patients with mHSPC with the following take-home points:
- In the ARASENS subpopulation of Chinese patients with mHSPC, darolutamide + ADT + docetaxel showed favorable efficacy and safety vs placebo + ADT + docetaxel
- Overall survival and clinically relevant secondary endpoints favored darolutamide vs placebo, and the incidences of treatment-emergent adverse events were similar in the two treatment groups
- These results are consistent with the findings reported for the overall population in ARASENS
Presented by: Shanshan Wang, PhD, Fudan University Shanghai Cancer Center, Shanghai, China
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
Reference:


