(UroToday.com) Dr. Patrizia Giannatempo reports on the impact of histology on the efficacy and safety of pembrolizumab monotherapy (pembro) for advanced urothelial carcinoma in the Phase 3 KEYNOTE-045 and KEYNOTE-361 trials.
First, she starts by reviewing the data from KEYNOTE-361 (NCT02853305) and KEYNOTE-045 (NCT02256436), which demonstrated the efficacy of pembrolizumab monotherapy in the treatment of advanced UC. In KEYNOTE-361, pembro ± platinum-based chemotherapy (chemo) vs platinum-based chemo was assessed and established the antitumor of pembo in the 1L setting - although it did not meet its dual primary endpoint of overall survival and progression free survival, the efficacy of pembrolizumab monotherapy appeared comparable to previous analysis in cisplatin ineligible patients. In KEYNOTE-045, a study of pembro vs investigator’s choice chemo in the second line setting, pembro again was demonstrated to provide durable antitumor activity.
The study design of both studies is reviewed below:

Currently, pembrolizumab is approved in the 1L setting for as monotherapy for patients with metastatic urothelial carcinoma who are platinum ineligible or for those whose decrease progressed following platinum-based chemotherapy.
In this study, they focus more on the evaluating efficacy and safety of pembro monotherapy stratified by UC histology. patients in both KEYNOTE-361 and KEYNOTE-045 we're required to have transitional cell carcinoma (tcc) as the predominant Histology but both pure TCC and mixed histologies were allowed. She notes that it remains unclear if pure TCC and mixed TCC have similar benefit to immunotherapy.
Endpoints for this exploratory analysis were ORR, DOR, and PFS per RECIST v1.1 by central radiology assessment, and OS and safety in pts with pure TC or mixed predominant TC allocated to receive pembro monotherapy. Efficacy was evaluated in the intent to treat population while safety was evaluated in all treated patients.
A total of 307 randomly assigned pts (280 [91.2%] pure TC histology; 27 [8.8%] mixed predominant TC histology) from KEYNOTE-361 and 268 pts (186 [69.4%] pure TC histology; 82 [30.6%] mixed predominant TC histology) from KEYNOTE-045 were included.
Demographics summarized below:
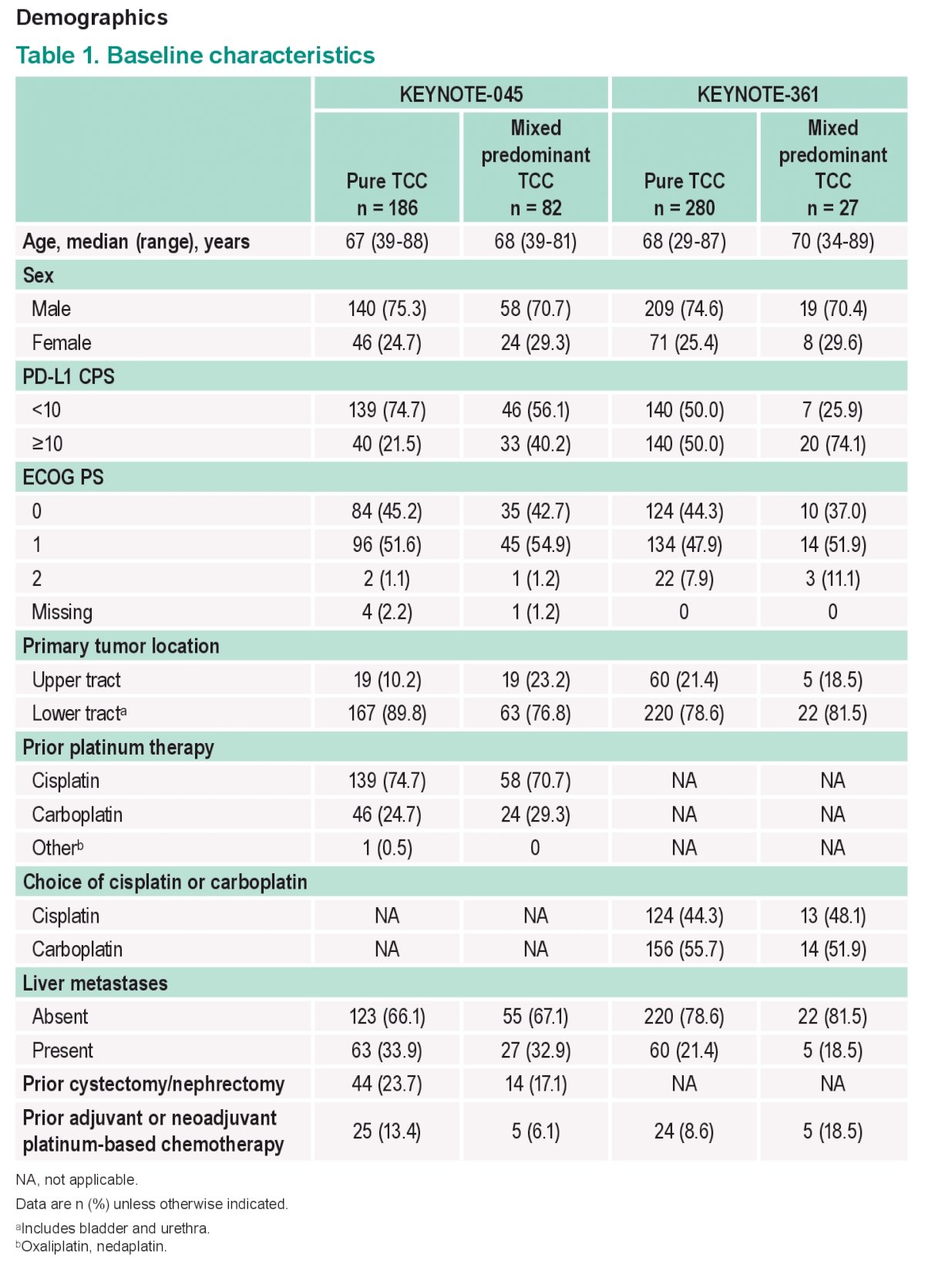
Median follow-up for both studies was ≥32 mo.
Efficacy outcomes are summarized below:
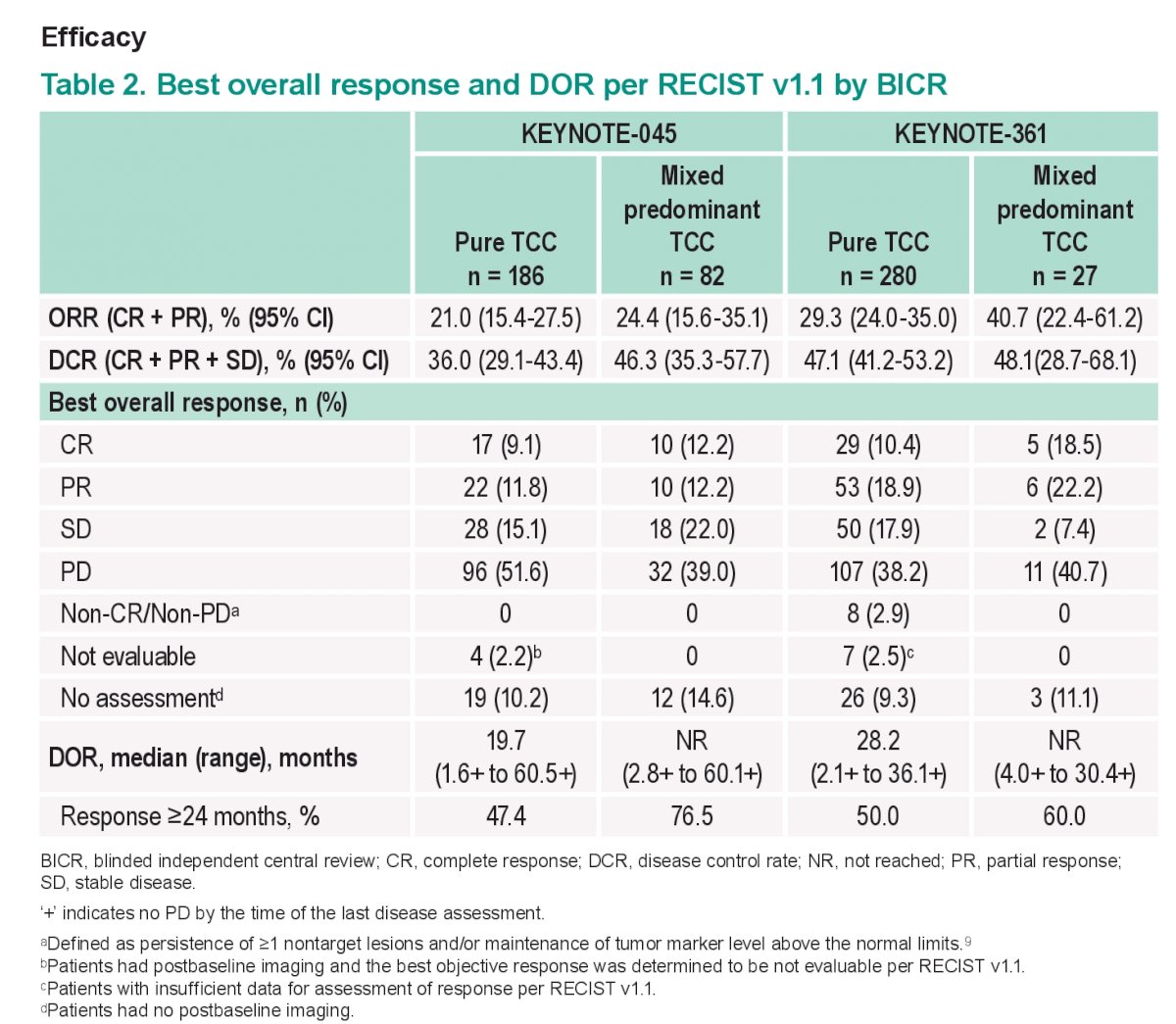
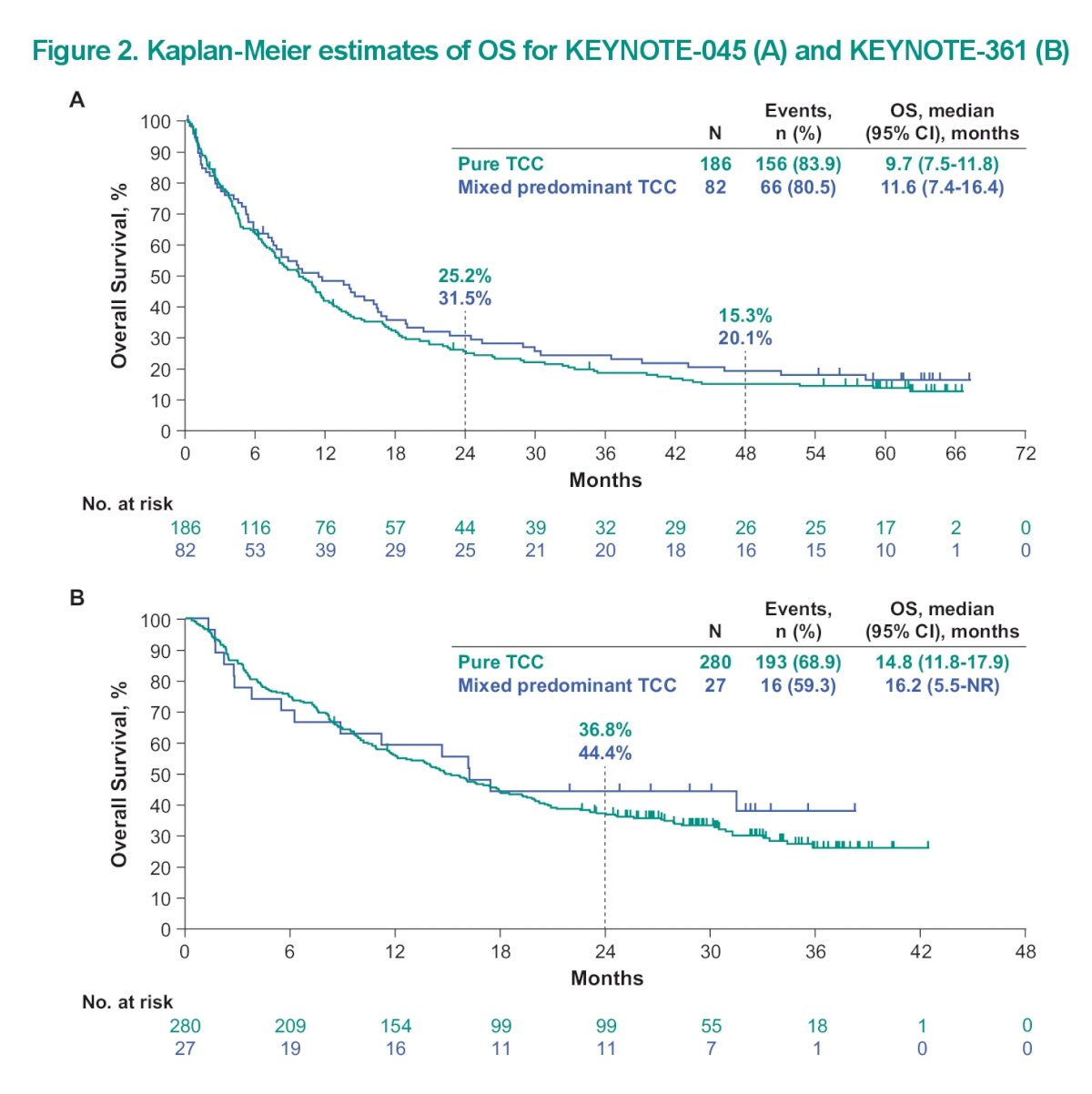
The key take home points here are that the key efficacy outcomes (PFS, ORR, DOR, and OS) for pembro were generally similar in patients with mixed predominant TC histology and pts with pure TC histology. therefore it would appear that a mixed Histology did not result in significantly different outcomes.
This is reinforced by the overall survival Kaplan Meyer curve seen below:
As for safety, in treated pts, grade 3-5 treatment-related AEs occurred at similar rates in KEYNOTE-361 (17.3% [48/277] pure TC; 18.5% [5/27] mixed predominant TC) and KEYNOTE-045 (16.9% [31/183] pure TC; 17.3% [14/81] mixed predominant TC).
Again from a safety profile standpoint, the Histology did not seem to impact the adverse event profile.
Based on this analysis from the two KEYNOTE trials, mixed histology (with predominant TCC) tumors should be treated the same as pure TCC. however, we cannot comment on patients with pure variant Histology, as they may behave differently.
Clinical trial information: NCT02256436; NCT02853305.
Presented by: Patrizia Giannatempo, MD, IRCCS Foundation National Cancer Institute, Milan, Italy
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


