(UroToday.com) Arun Azad presents the full safety analysis from the TALAPRO-2 study, a randomized controlled trial comparing enzalutamide monotherapy to enzalutamide + talazoparib as a first line therapy for mCRPC.
The initial results of TALAPRO-2 were presented earlier this year at GU ASCO. As a reminder, at median follow up of nearly 25 months in both arms, the primary endpoint of rPFS via blinded independent review demonstrated a 37% risk of progression or death in the patients receiving talazoparib and enzalutamide, with 151 events in the experimental and 191 events in the control arm. Median rPFS was not yet reached with the addition of talazoparib as compared to 21.9 months (95% CI 16.6-25.1). The HR for rPFS was 0.63 (0.51-0.78) with p<0.001. The benefit was consistently reported in investigator-reported data as well. Objective response rates via BICR showed higher ORR and complete response rates in the experimental arm with an impressive CR rate of 37.5% (vs 18.2%). Overall survival data were reported but were not mature or yet suggestive of significance. Importantly, this was for a population unselected for homologous recombination repair gene alterations (all-comers population).
As the authors note, TALAPRO-2 is an ongoing phase III trial.
In today’s presentation, Dr. Azad details the safety profile of TALA + ENZA.
The all-comers (unselected for homologous recombination repair gene alterations) safety population included 398 pts in the TALA + ENZA arm. Data cutoff was August 16, 2022. The median treatment duration of TALA was 19.8 months. At study entry, 49% already had grade 1-2 anemia.
All-cause any-grade TEAEs were observed in 98.5% of pts. Overall, 19.1% of pts discontinued TALA due to TEAEs. The incidence of all-cause Hematologic (Figure 1A) and non-hematologic (Figure 1B) TEAEs are shown below:
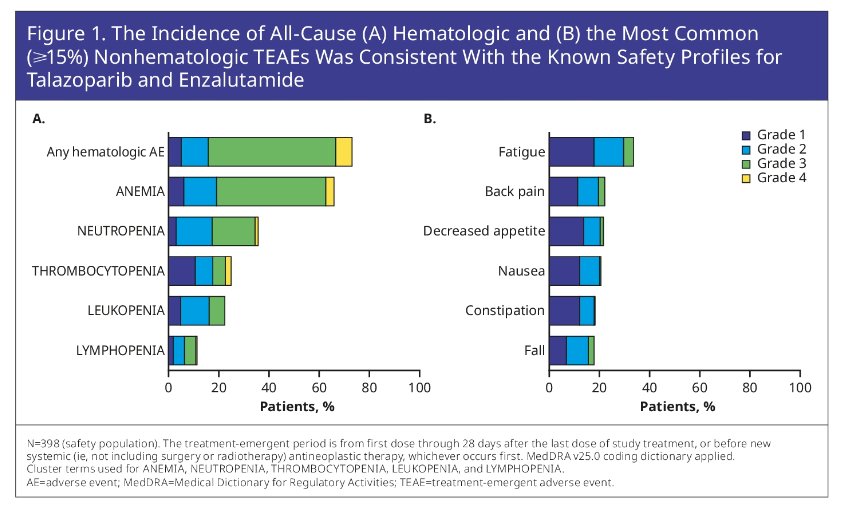
A similar incidence of all cause adverse events was observed in the HRR-deficient only cohort (see abstract number 5004).
Of note, most adverse events occurred early in the treatment time course, usually within the first 6 months – as seen below:
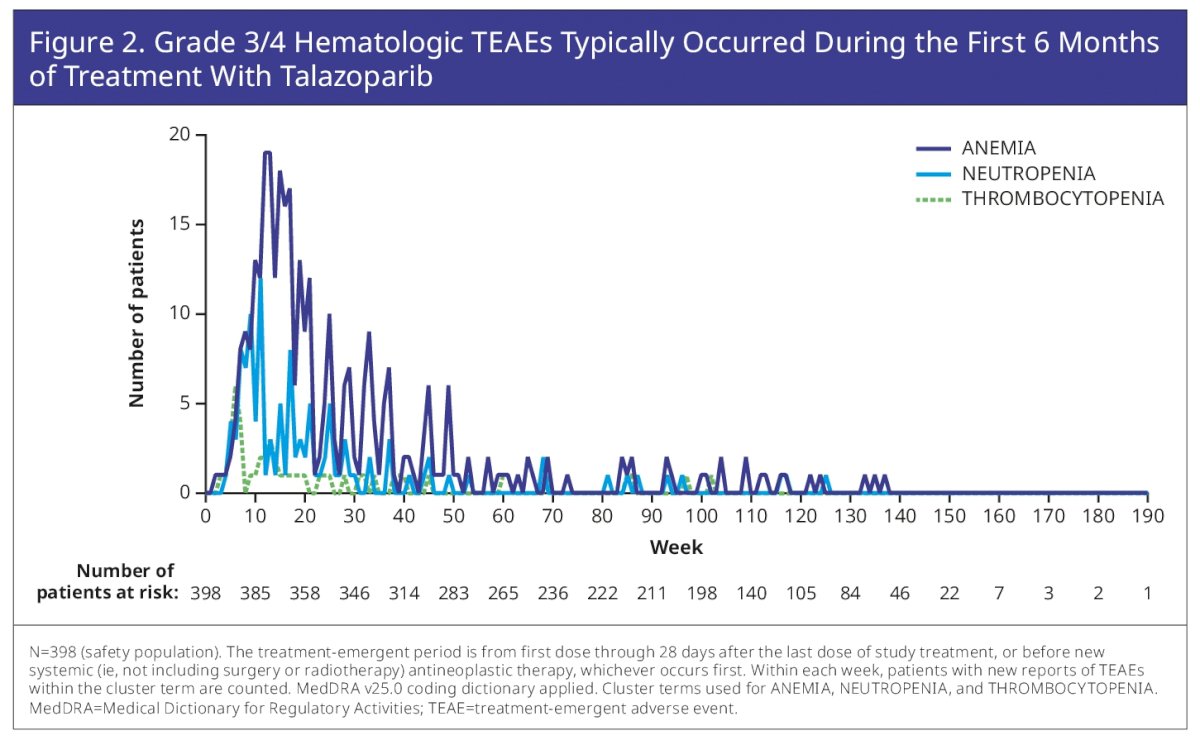
Specifically focused on the hematologic side effects, to ensure optimal dosing of TALA at the individual level, the protocol did not require dose modification of TALA until anemia was grade (G) ≥3. Median time to onset of first G≥3 anemia was 3.3 months. At study entry, 49.0% had G1–2 anemia. 43.2% had anemia leading to dose reduction (with or without transfusion) and 8.3% discontinued TALA due to anemia. Despite dose reduction, the relative dose intensity of talazoparib remained >80% (approx. 0.4 mg/day).

Based on all the above, Dr. Azad and colleagues concluded that the addition of TALA to ENZA did not add significant adverse event profile burden; what was increased was generally manageable, with dose modifications of TALA and/or standard supportive care. Anemia was the most common TEAE and led to discontinuation of TALA in 8.3% of pts. G≥3 hematologic AEs typically occurred within 6 months of starting therapy and resolved in <1 month. A detailed understanding of the onset, duration, and severity of AEs, as well as the impact of dose adjustments in the TALAPRO-2 study, will optimize the management of pts receiving TALA + ENZA for mCRPC.
Ultimately, if the combination of talazoparib with enzalutamide is approved, it has the potential to become a first-line treatment option for patients with mCRPC and HRR gene alterations.
Presented by: Arun Azad, MD, Peter MacCallum Cancer Centre, Melbourne, AustraliaWritten by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Agarwal N, Azad A, Shore ND, Carles J, Fay AP, Dunshee C, Karsh LI, Paccagnella ML, Santo ND, Elmeliegy M, Lin X, Czibere A, Fizazi K. Talazoparib plus enzalutamide in metastatic castration-resistant prostate cancer: TALAPRO-2 phase III study design. Future Oncol. 2022 Feb;18(4):425-436. doi: 10.2217/fon-2021-0811. Epub 2022 Jan 26. PMID: 35080190.


