(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers oral abstract session. Dr. David Olmos presented the results of an analysis evaluating the association between presence of somatic/germline homologous recombination repair (HRR) gene alterations and outcomes in metastatic castrate-resistant prostate cancer (mCRPC) patients receiving 1st line treatment, stratified by BRCA mutational status.
Pathogenic alterations in DNA damage repair (DDR) genes causing loss of function have been described in up to 30% of mCRPC patients.1,2 Defects in different DDR genes/pathways have been linked to potential enhanced responses to novel targeted agents, including PARP inhibitors alone or in combination with androgen receptor signaling inhibitors.3 Deleterious germline BRCA2 mutations have been consistently associated with worse survival outcomes across various PCa stages, including mCRPC. The prognostic significance of other germline DDR mutations remains less clear. Indirect comparisons have suggested an adverse prognostic role for somatic BRCA2 and/or DDR defects. Importantly, the predictive value of such mutations for the treatment of mCRPC patients needs to be further clarified.
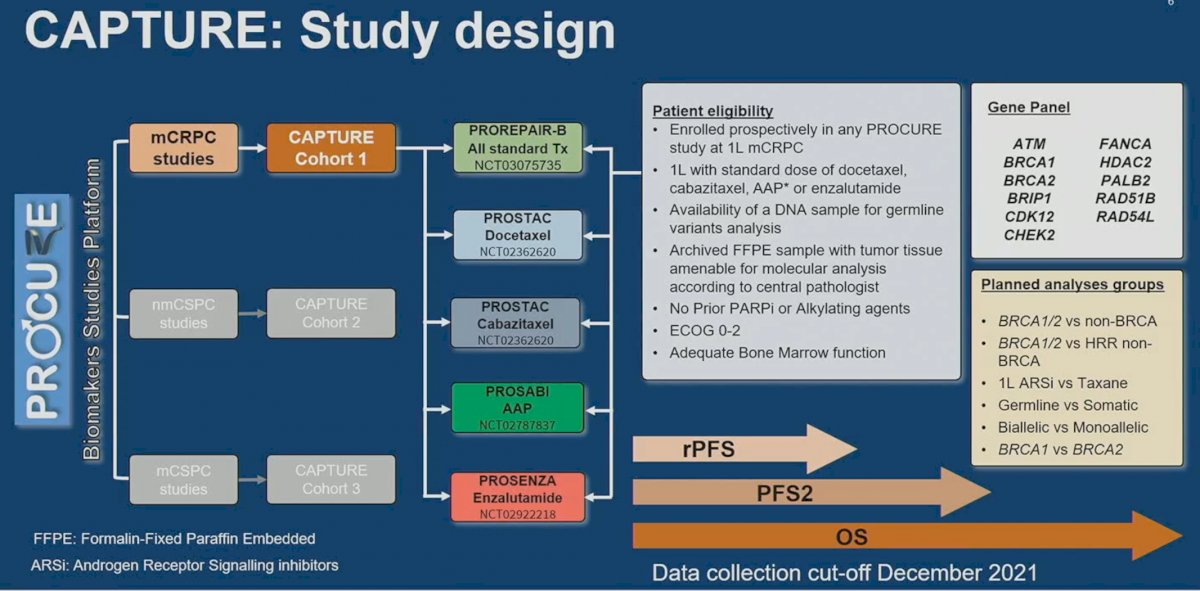
Using the PROCURE Biomarkers Studies Platform, CAPTURE Cohort 1 included patients from mCRPC studies, including:
- PROREPAIR-B
- PROSTAC (docetaxel and cabazitaxel)
- PROSABI (abiraterone acetate + prednisone)
- PROSENZA (enzalutamide)
Patients were eligible if they were enrolled prospectively in any PROCURE study of 1st line mCRPC treatment, which included docetaxel, cabazitaxel, abiraterone acetate/prednisone, or enzalutamide. Patients had to have a DNA sample available for germline variants analysis, archived FFPE sample with tumor tissue amenable for molecular analysis according to central pathologist. Patients did not receive prior PARP inhibitors or alkylating agents and had adequate bone marrow function and ECOG performance status. The evaluable gene panel included: ATM, BRCA1/2, BRIP1, CDK12, CHECK2, FANCA, HDAC2, PALB2, RAD51B, and RAD54L. Evaluable study outcomes included radiographic progression-free survival (rPFS), progression-free survival 2 (PFS2), and overall survival (OS).
DNA from blood leukocytes and from archived tumor FFPE samples were analyzed for each patient. Patients harboring any pathogenic/likely-pathogenic variant, heterozygous or homozygous loss, or any combination were hierarchically classified:
- BRCA1/2 > HRR non-BRCA > non-HRR
- Germline > Somatic > None
- Bi-allelic > Mono-allelic > None
The outcomes of interest were analyzed using inverse probability weighting methods to correct baseline imbalances.
This analysis included a total of 729 patients. BRCA1/2 mutations were present in 13.2% of patients, with 9.8% and 3.4% having somatic and germline mutations, respectively. Mono-allelic and bi-allelic BRCA1/2 mutations were present in 5.8% and 7.4% of patients, respectively. HRR non-BRCA mutations were present in 17.4% of patients, with 13.4% and 4% somatic and germline, respectively. Mono-allelic mutations were more common for HRR non-BRCA mutations (10.1% versus 7.3%).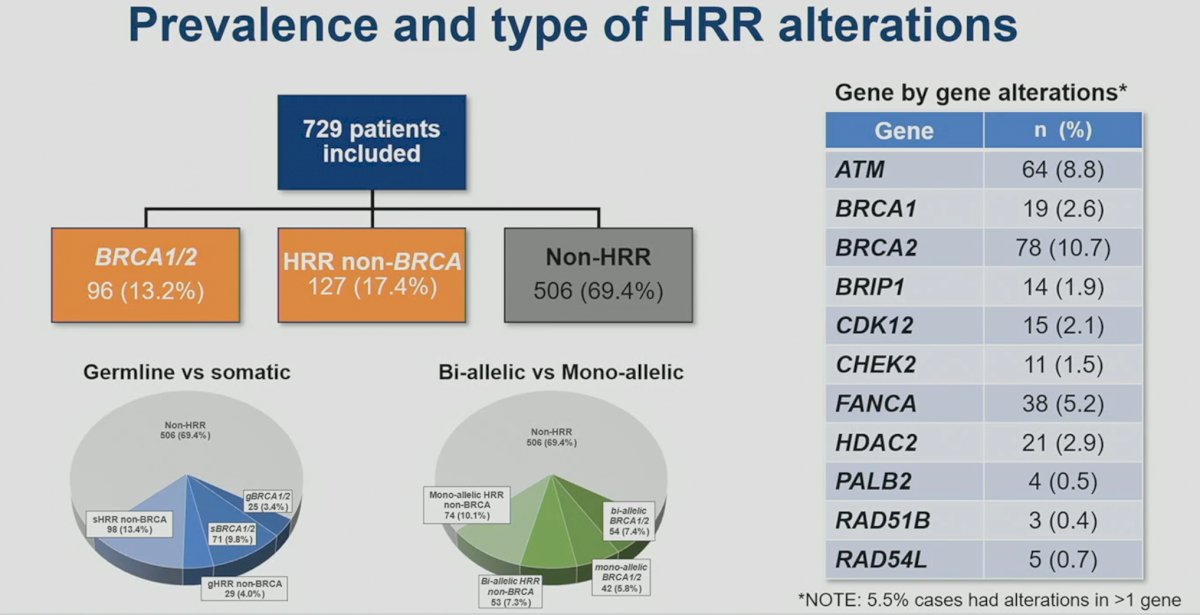
Overall baseline characteristics were well-balanced between the BRCA1/2, the HRR non-BRCA mutated, and non-HRR mutated cohorts. 
Looking at treatment exposure by subgroup, Dr. Olmos noted that BRCA1/2 patients with mCRPC were more likely to receive 2nd line treatments (92% versus 79% for HRR non-BRCA and non-HRR patients).
On propensity score weighted Kaplan Meier curves, BRCA1/2 mutated patients had worse rPFS outcomes compared to non-HRR (HR: 1.70, 95% CI: 1.32 – 2.19, p<0.001) and HRR non-BRCA mutated patients (HR: 1.34, 95% CI: 0.98 – 1.81, p=0.06).
Similarly, BRCA1/2 mutations were prognostic of worse PFS2 outcomes compared to non-HRR (HR: 1.81, 95% CI: 1.44-2.27, p<0.0001) and HRR non-BRCA mutated patients (HR: 1.42, 95% CI: 1.07 – 1.88, p=0.014).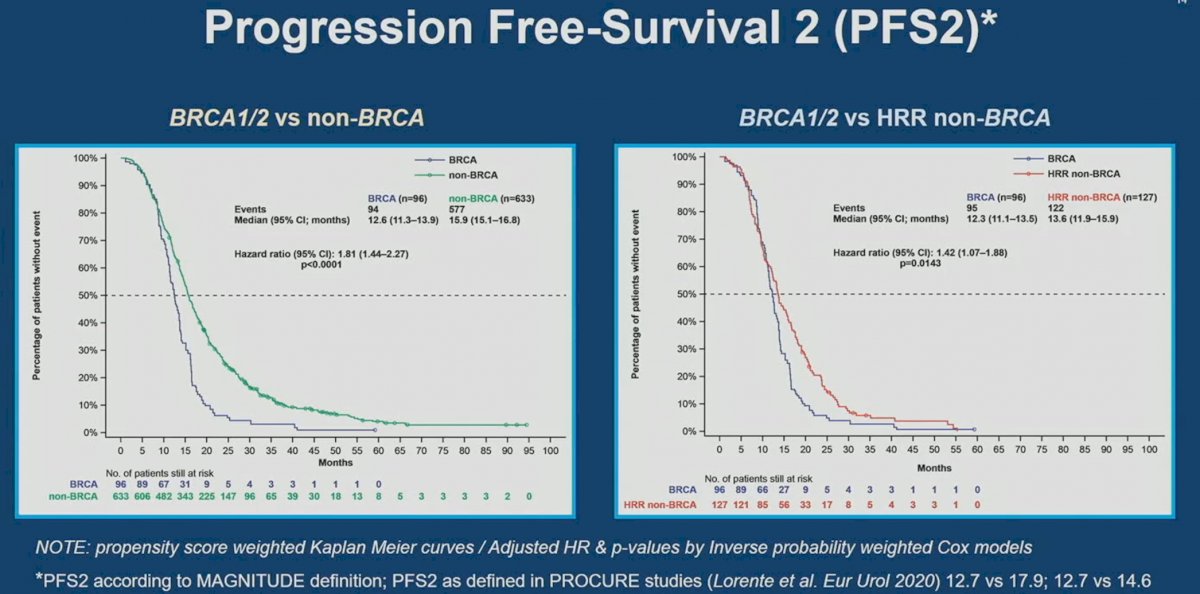
Significantly, OS was worse in BRCA1/2 mutated patients compared to non-HRR (HR: 1.95, 95% CI: 1.55 – 2.45, p<0.001) and HRR non-BRCA mutated patients (HR:1.40, 95% CI: 1.06 – 1.84, p=0.017).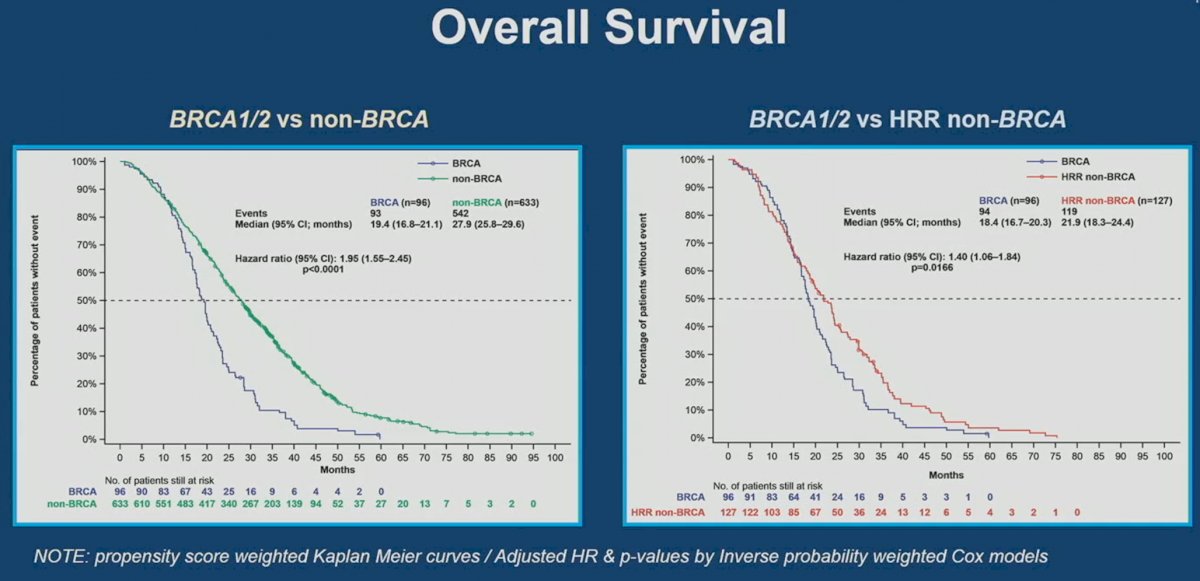
The outcomes by subgroup are summarized below. Overall, it appears that the outcomes of mCRPC patients treated in the 1st line setting are worst if they had BRCA1/2 mutations, followed by HRR non-BRCA, the overall cohort, and then non-HRR mutated patients.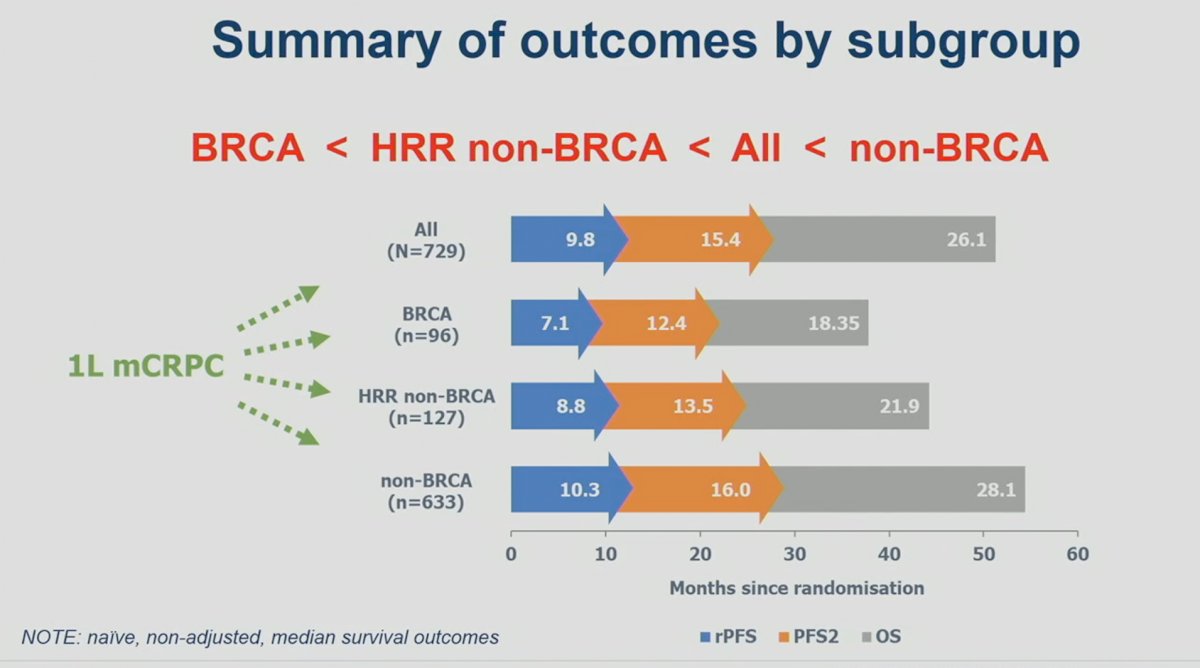
Exploratory analysis within the BRCA1/2 subgroup demonstrated that patients had similar rPFS, PFS2, and OS outcomes, irrespective of whether the BRCA1/2 mutation was germline versus somatic or mono- versus bi-allelic.
Dr. Olmos concluded that:
- Both germline and somatic BRCA1/2 alterations are associated with shorter rPFS, PFS2 and OS in mCRPC patients treated with either an androgen receptor signaling inhibitor or taxanes in the first-line setting.
- BRCA1/2 alterations are associated with worse mCRPC outcomes compared to other HRR alterations
- These poor outcomes are not related to a less frequent exposure to active mCRPC treatments
- Exploratory analysis in the BRCA1/2 subgroup, although limited, suggested that no significant differences were observed in rPFS, PFS2, and OS between those treated with taxanes versus novel hormone therapies, germline versus somatic, bi-allelic versus mono-allelic, or BRCA2 versus BRCA1 mutations
- These results further support the importance of screening germline and somatic BRCA1/2 alterations for a more precise patient-centered approach
Presented by: David Olmos, MD, PhD, Medical Oncologist, Facultad de Medicina, Universidad de Málaga, Málaga, Spain
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:- Robinson D, Van Allen EM, Wu Y, et al. Integrative clinical genomics of advanced prostate cancer. Cell, 2015;161(5):1215-1228.
- Lozano R, Castro E, Aragon IM, et al. Genetic aberrations in DNA repair pathways: a cornerstone of precision oncology in prostate cancer. Br J Cancer, 2021;124(3):552-563.
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence 2022.EVIDoa2200043


