(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Eva Lengyelova discussing 64Cu-SAR-bisPSMA PET imaging in patients with confirmed prostate cancer. PSMA is a type II transmembrane glycoprotein that is overexpressed in prostate cancer. Recently, several new 68Ga- and 18F-based PSMA-targeted PET agents have been approved by the FDA to detect prostate cancer, which offer improved specificity compared to traditional imaging. However, these products exhibit low sensitivity for prostate cancer potentially due to low tumor uptake and retention, or limitations of the short half-life of the isotopes being used. 64Cu-SAR-bisPSMA is specifically designed to overcome these issues due to:
- The targeting moiety having two PSMA-targeting functional groups which have exhibited increased tumor uptake and retention
- The copper-64 isotope, which has a longer half-life of 12.7 hours, longer shelf-life, greater flexibility for patient scheduling, and the ability to image at later time points, which has previously been shown to detect additional lesions
- 64Cu has a shorter positron range (0.56 mm), leading to improved scan resolution
PROPELLER (NCT04839367) was a prospective, phase 1, multi-center, blinded review, dose-ranging study evaluating safety and preliminary efficacy of 64Cu-SAR-bisPSMA PET in patients with known primary prostate cancer. The aims of PROPELLER were to: (i) determine the safety and tolerability of 64Cu-SAR-bisPSMA, (ii) determine the ability of 64Cu-SAR-bisPSMA PET to detect primary prostate cancer, (iii) assess image quality at 100 MBq, 150 MBq and 200 MBq dosages of 64Cu-SAR-bisPSMA, and (iv) explore how 64Cu-SAR-bisPSMA compares to 68Ga-PSMA-11 PET, a standard of care radiotracer for imaging PSMA-positive lesions in prostate cancer.
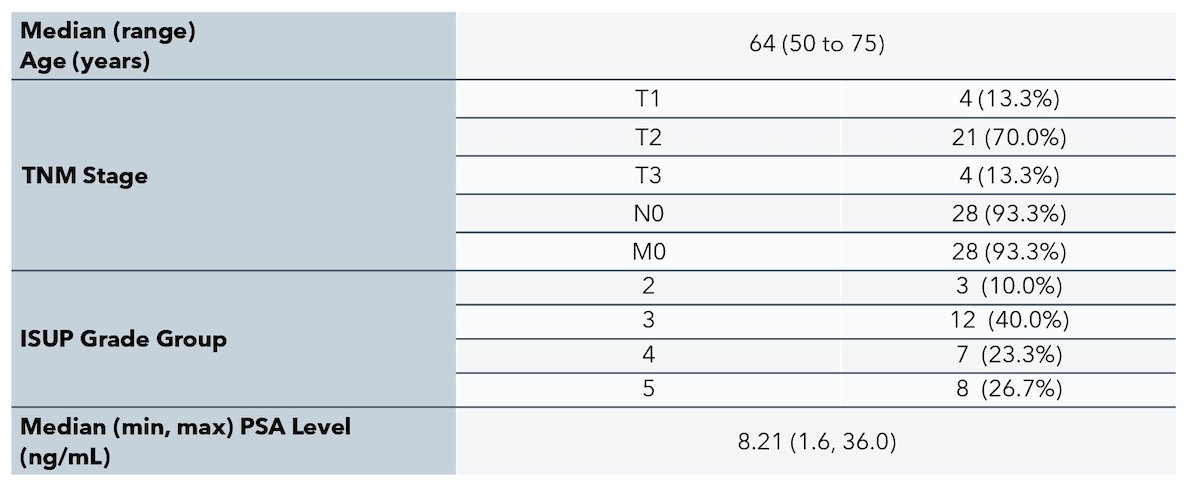
PROPELLER investigated 64Cu-SAR-bisPSMA in 30 men with untreated, histopathologically proven, primary prostate cancer with intermediate- to high-risk features. The demographics of these patients is as follows:
At screening, patients completed 68Ga-PSMA-11 PET/CT at 1 hour post dose. Following enrollment, patients received either 100 MBq, 150 MBq or 200 MBq of 64Cu-SAR-bisPSMA, followed by PET/CT at 2-4 hour post dose. Safety was evaluated before and after 64Cu-SAR-bisPSMA dosing. The 68Ga-PSMA-11 and 64Cu-SAR-bisPSMA PET/CT scans were evaluated by two independent, blinded central readers for image quality, prostate cancer detection, and intensity of tracer uptake in lesions (SUVmax and tumor-to-background ratio).
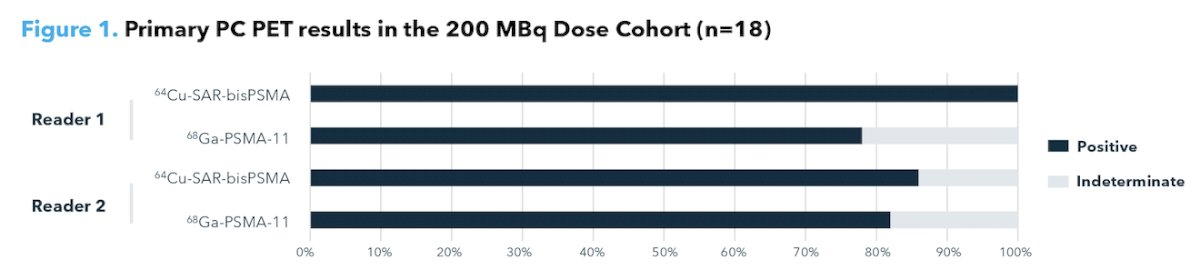
A single, grade 1 64Cu-SAR-bisPSMA-related adverse event was reported in the 200 MBq cohort. There were no clinically significant changes in any laboratory measures or ECGs. Both readers scored 200 MBq of 64Cu-SAR-bisPSMA as the dose providing the highest image quality. Interval between the 68Ga-PSMA-11 and 64Cu-SAR-bisPSMA scans was 2-50 days (median 20.5 days). In this 200 MBq cohort, 64Cu-SAR-bisPSMA and 68Ga-PSMA-11 were able to detect primary prostate cancer in 100% and 77.8% of patients for Reader 1 and 85.7% and 83.3% of patients for Reader 2, respectively:
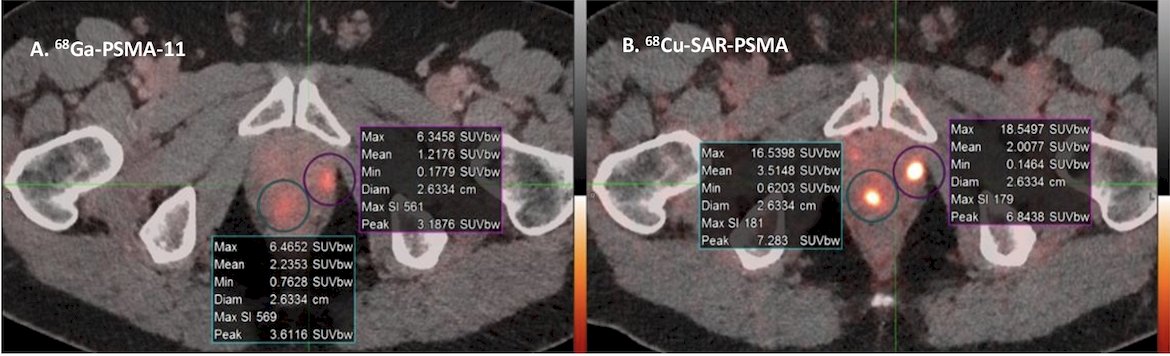
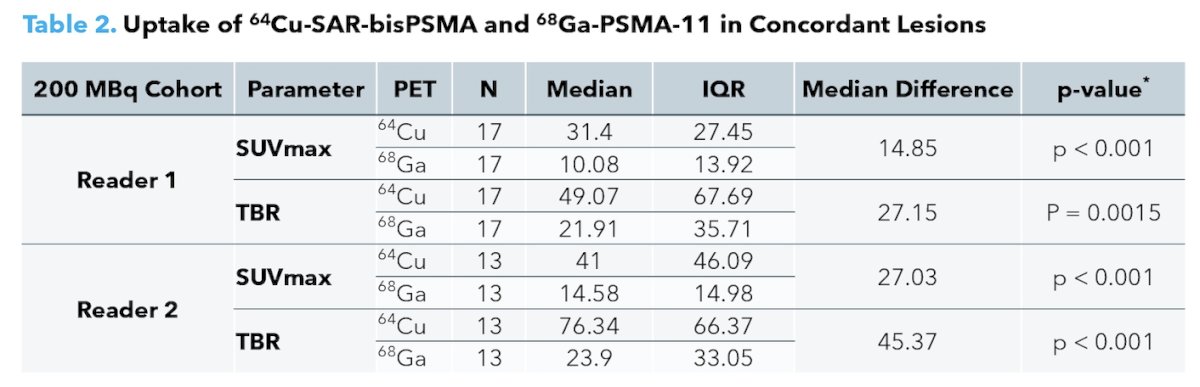
Concordant lesions on 64Cu-SAR-bisPSMA and 68Ga-PSMA-11 PET/CT showed 2.5-3 times higher uptake on 64Cu-SAR-bisPSMA compared to 68Ga-PSMA-11 in all parameters assessed:
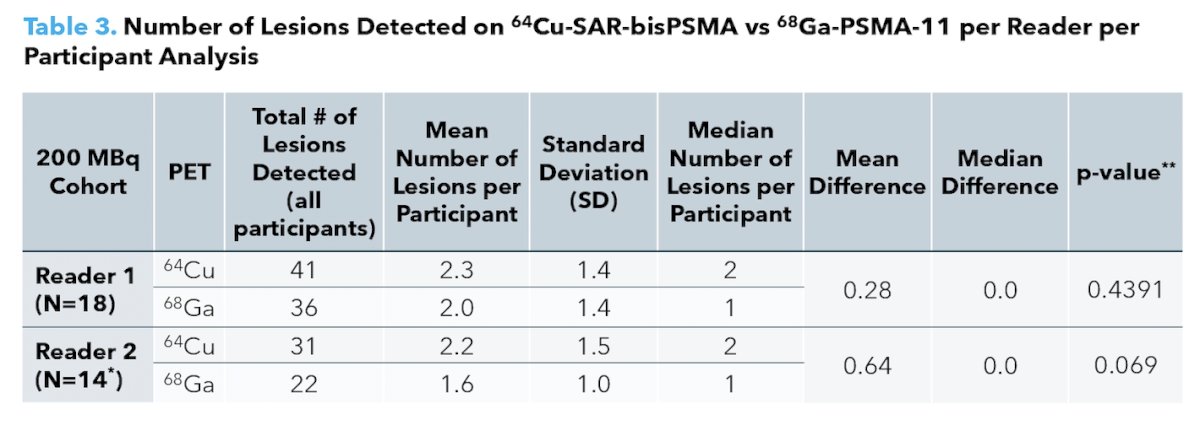
Reader 1 noted 38.9% of patients as having more lesions detected by 64Cu-SAR-bisPSMA versus 22.2% of patients as having more lesions detected by 68Ga-PSMA-11 PET/CT, while Reader 2 noted 42.9% of patients as having more lesions detected by 64Cu-SAR-bisPSMA versus 14.3% of patients as having more lesions detected by 68Ga-PSMA-11 PET/CT. In total, there were more lesions identified on 64Cu-SAR-bisPSMA than on by 68Ga-PSMA-11 PET/CT according to both readers:
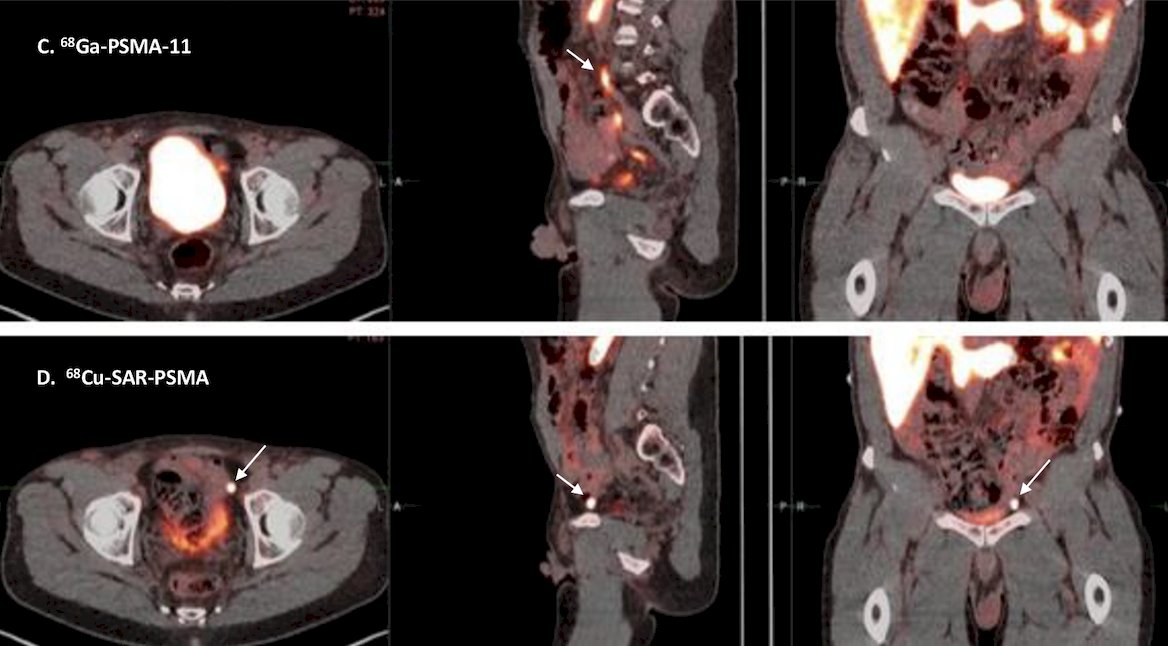
64Cu-SAR-bisPSMA consistently showed higher uptake compared to 68Ga-PSMA-11:
- SUVmax:
- Reader 1 median: 31.40 [IQR 27.45] vs 10.08 [IQR 13.92], p < 0.001
- Reader 2 median: 41.00 [IQR 46.09] vs 14.58 [IQR 14.98], p < 0.001
- Tumor-to-Background Ratio:
- Reader 1 median: 49.07 [IQR 67.69] vs 21.91 [IQR 35.71], p = 0.0015 Reader 2 median: 76.34 [IQR 66.37] vs 23.90 [IQR 33.05], p < 0.001
Dr. Lengyelova concluded her presentation discussing 64Cu-SAR-bisPSMA PET imaging in patients with confirmed prostate cancer by highlighting the following take-home points:
- 64Cu-SAR-bisPSMA was safe and effective for detecting PSMA-expressing lesions
- 200 MBq of 64Cu-SAR-bisPSMA was determined as the optimal dose for future trials
- 64Cu-SAR-bisPSMA PET/CT showed a greater number of lesions and lesions exhibited significantly higher uptake when compared to 68Ga-PSMA-11 in men being with intermediate- to high-risk prostate cancer
- Further studies to evaluate 64Cu-SAR-bisPSMA as an imaging agent in patients with biochemical recurrence of prostate cancer are underway
Presented by: Eva Lengyelova, Clarity Pharmaceuticals, Sydney, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


