(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Alexandra Sokolova discussing the final analysis of a trial assessing concurrent chemo-hormonal therapy of enzalutamide and cabazitaxel in patients with mCRPC. Previously, this group reported the PSA response rate and toxicity data of a phase 1/2 single-arm, multi-institutional trial to examine the efficacy and safety of co-administration of enzalutamide + cabazitaxel in mCRPC without prior chemotherapy given in the mCRPC setting. They found that full doses of enzalutamide (160 mg daily) and cabazitaxel (25 mg/m2) were tolerable and that 80% of patients had PSA decline ≥50%. At the 2023 ASCO annual meeting, Dr. Sokolova and colleagues reported the final analysis of objective response rate, radiographic progression free survival, and overall survival.
Dr. Sokolova calculated the objective response rate (complete response/partial response) and 95% confidence intervals using the Clopper-Pearson Exact Method. They determined response according to RECIST 1.1 for measurable disease and Prostate Cancer Working Group 2 criteria for non-measurable (bone) disease. Median radiographic progression free survival (time from the study entry to the time of confirmed progression (radiographic or clinical) or death) and overall survival (time from the study entry to death) with 95% confidence intervals were estimated using the Kaplan-Meier method with a data cutoff of June 1, 2022.
There were 37 patients consented and 35 which were included in the efficacy analyses (one withdrew consent and one was lost to follow up before efficacy assessment). Of note, 7/35 (20%) had prior exposure to chemotherapy given for mHSPC and 9/35 (25%) had prior exposure to abiraterone, including 2/35 (25.7%) with prior exposure to both chemotherapy and abiraterone. There were 28 patients that had at least one on-study trial imaging study and were evaluable for objective response rate, which is highlighted in the following table:
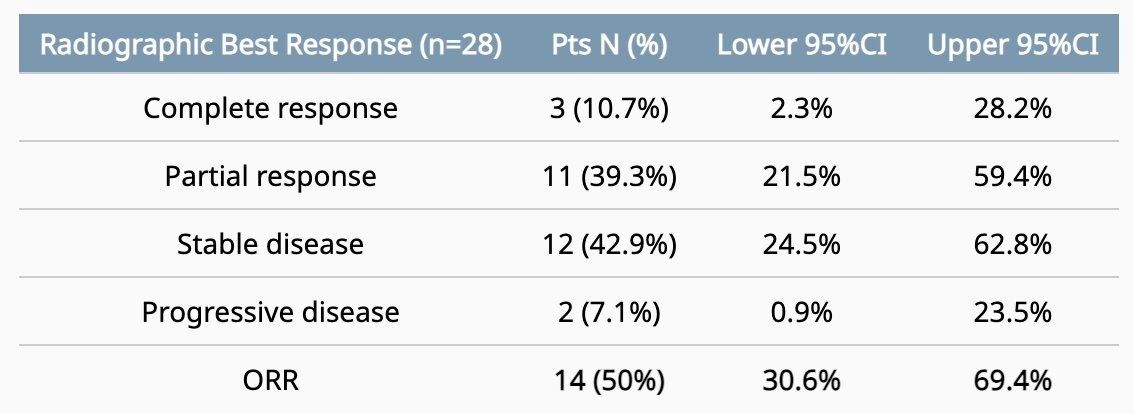
After a median follow-up of 23.7 (range 4.9 to 62.4) months, median overall survival was 25.1 months (95% CI 19.4 - 37.6 months), median PSA progression free survival was 11.9 months (95% CI 9.2 - 15.4), and median radiographic progression free survival was 22.2 months (95% CI 13.6 - 25.2). Pharmacokinetic assessments showed that enzalutamide decreased cabazitaxel levels: cabazitaxel (monotherapy) Cmax 178.9ng*h/ml versus cabazitaxel (in the presence of enzalutamide) Cmax 85.5 ng*h/ml (p<0.05).
Dr. Sokolova concluded her presentation discussing the final analysis of a trial assessing concurrent chemo-hormonal therapy of enzalutamide and cabazitaxel in patients with mCRPC by highlighting the following take-home points:
- The combination of enzalutamide + cabazitaxel in this heterogeneous mCRPC population, which included about a quarter of patients with prior chemotherapy and abiraterone exposure, resulted in an OS of 25.1 months comparing favorably with the overall survival of 25.2 months seen in the FIRSTANA trial [1] of single agent cabazitaxel in docetaxel naïve men, 15.1 months post chemotherapy in the TROPIC trial [2], and 13.6 months in the post-docetaxel, post- abiraterone in the CARD trial [3]
- Of note, the radiographic progression free survival in this trial was 22.2 months compared to 5.1 months in FIRSTANA suggesting improved efficacy; however cross-trial comparisons are discouraged due to several confounders
- As such, chemo-hormonal therapies and prognostic/predictive biomarkers warrant further study in mCRPC
Presented by: Alexandra Sokolova, MD, OHSU Knight Cancer Institute, Portland, OR
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Oudard S, Fizazi K, Sengelov L, et al. Cabizitaxel versus Docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: A randomized phase III Trial-FIRSTANA. J Clin Oncol 2017;35(28):3189-3197.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010;376(9747):1147-1154.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019 Dec 26;381(26):2506-2518.


