(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Georges Gebrael discussing the impact of synchronous vs metachronous metastasis at presentation on survival in patients with mCRPC treated with androgen receptor signaling inhibitors. Patients with synchronous metastatic castration-sensitive prostate cancer (mCSPC) appear to have worse survival outcomes and earlier time to develop castration resistance than patients with metachronous mCSPC.1 However, the impact of the initial timing of metastasis on survival in the mCRPC setting is less clear. As such, the objective of this study was to assess the correlation between the synchronous/metachronous metastatic disease presentation and survival in real-world newly diagnosed mCRPC patients.
In this study, patient-level data were retrospectively collected. Patients were eligible if they had confirmed diagnosis of mCRPC, treatment with androgen receptor signaling inhibitors (abiraterone or enzalutamide) as first-line therapy for mCRPC, and no prior exposure to androgen receptor signaling inhibitors in the mCSPC or localized states. The disease was considered synchronous if distant metastasis was diagnosed within 90 days of the initial prostate cancer diagnosis. The study endpoints included progression-free survival, defined from the start of therapy for first-line mCRPC to progression (per PCWG-2) or death from any cause. Overall survival was defined from the start of therapy for first-line mCRPC to the date of death from any cause or censored at the last follow-up. A second analysis assessed the effect of synchronous versus metachronous disease on overall survival in two patient populations receiving first-line treatment in mCRPC: those who received ADT intensification and those who did not, in the context of mCSPC.
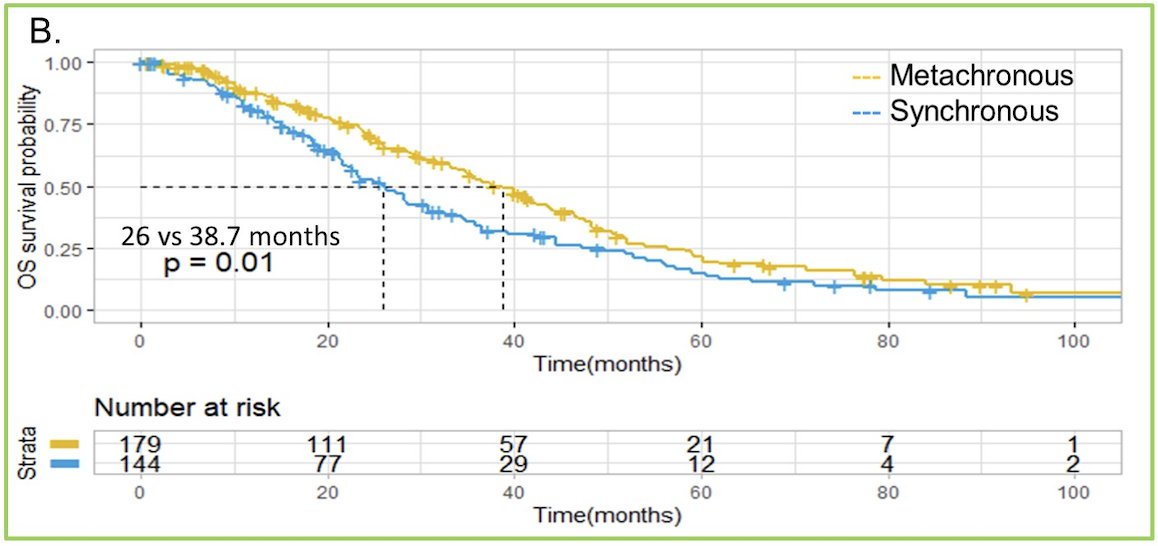
Between 2011-2022, there were 323 patients (144 synchronous and 179 metachronous) eligible and included in the analysis. Synchronous and metachronous patients had similar baseline characteristics: median PSA (19.6 ng/mL vs 20.8 ng/ml), liver metastasis (9% vs 12.3%), median alkaline phosphatase (97 IU/L vs 95 IU/L), and ECOG performance > 1 (9% vs 3.9%). The complete baseline characteristics is as follows:
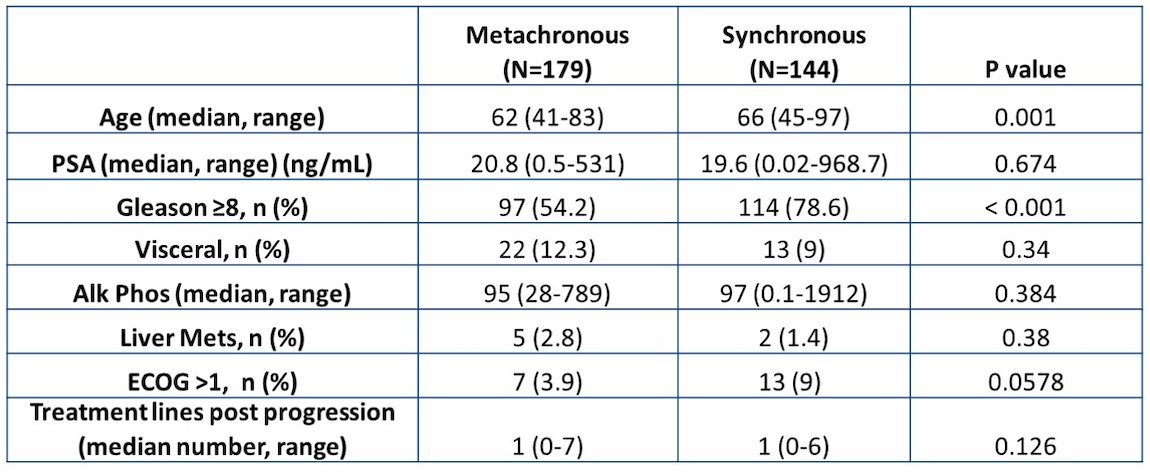
The difference in median progression-free survival was not statistically significant (8.2 vs. 10.0 months, HR 1.13, 95% CI 0.85-1.49, for synchronous and metachronous tumors):
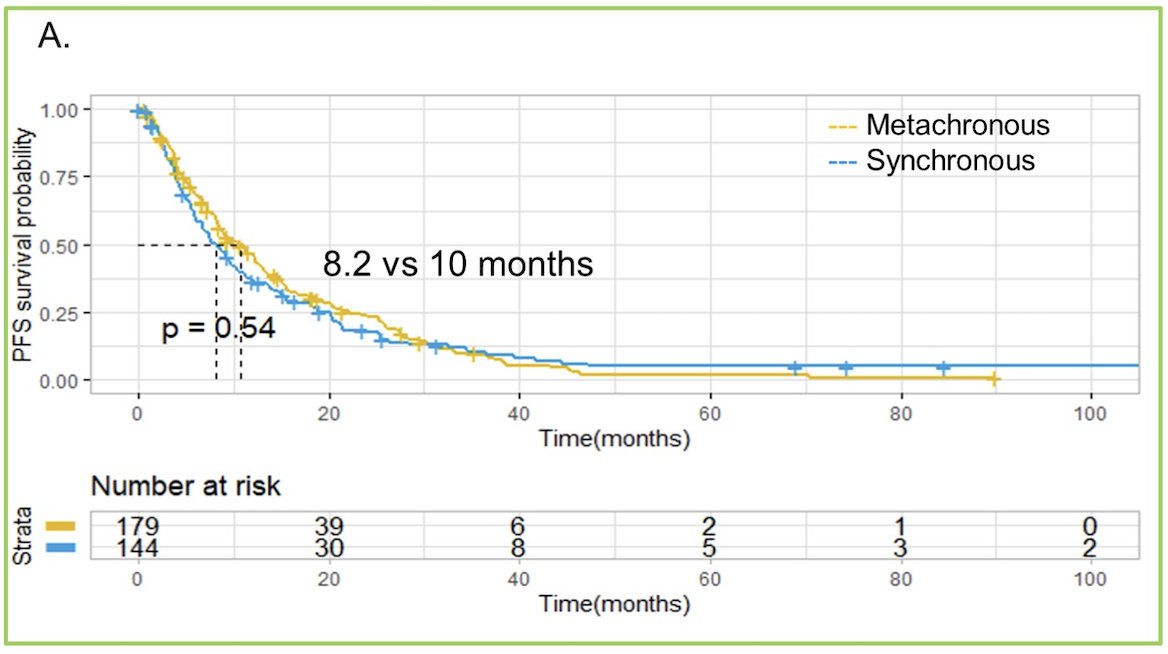
However, median overall survival was significantly shorter in patients with synchronous compared to metachronous (26 vs. 38.7 months, HR 1.51, 95% CI 1.11-2.08):
In multivariate analysis, the initial timing of metastasis remained an independent prognostic factor. In the second analysis, there were 368 patients who did not receive ADT intensification who were compared to 151 patients who did receive intensification. In patients who received ADT monotherapy, the median overall survival for metachronous patients was 37.5 months, compared to 24.8 months for synchronous patients (p = 0.008). For metachronous and synchronous patients who received ADT intensification, the median overall survival was 21.6 and 20.9 months, respectively, but the difference was not statistically significant (p = 0.189).
Limitations of this study include the retrospective design and single institution nature with a lack of external validation. Additionally, there are limited implications for the clinical practice in view of the evolved treatment landscape in the setting of mCSPC.
Dr. Gebrael concluded his presentation discussing the impact of synchronous vs metachronous metastasis at presentation on survival in patients with mCRPC treated with androgen receptor signaling inhibitors by highlighting the following take-home points:
- For the first time, this study reports the impact of the initial timing of metastasis on mCRPC prognosis in a real-world patient population with synchronous disease being associated with having a poor prognosis
- Initial timing of metastasis may be worth accounting for in future mCRPC clinical trials to explore its prognostic value further
- Following external validation, this information may be useful for patient counseling, prognostication, and the design and selection of stratification factors of future clinical trials in the new onset mCRPC setting
Presented by: Georges Gebrael, MD, Huntsman Cancer Institute at the University of Utah, Salt Lake City, UT
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: Final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021 Jul 10;39(20):2294-2303.


