(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Arun Azad discussing safety analyses from TALAPRO-2, a phase 3 trial assessing talazoparib + enzalutamide in metastatic castration-resistant prostate cancer (mCRPC). Despite recent approvals of new agents, mCRPC remains aggressive and the need for novel therapeutic strategies persists. Co-inhibition of the androgen receptor and PARP may result in enhanced benefit in the treatment of tumors regardless of alterations in DNA damage response genes involved either directly or indirectly in homologous recombination repair (HRR). The phase 3 TALAPRO-2 study demonstrated a clinically meaningful and statistically significant improvement in radiographic progression-free survival for 1st-line talazoparib + enzalutamide versus placebo + enzalutamide in men with mCRPC, unselected for homologous recombination repair gene alterations (all-comers population).1 At the ASCO 2023 annual meeting, Dr. Azad and colleagues detailed the safety profile of talazoparib + enzalutamide.
In this double-blind, randomized, placebo-controlled trial, men received talazoparib 0.5 mg or placebo, plus enzalutamide 160 mg once daily (QD). Patients were ≥18 yrs of age, had Eastern Cooperative Oncology Group performance status ≤1, were receiving ongoing ADT, and had no prior systemic therapy for mCRPC. Patients were recruited to two cohorts, an all-comers cohort that includes patients with and without HRR gene alterations, and an HRR-deficient only cohort. Reflective of a more real-world population, study patients were eligible to enter with lowered (>9 g/dL) hemoglobin levels, and the protocol did not require dose modification of talazoparib until anemia was >= grade 3. Treatment-emergent adverse events were evaluated, including type, severity, timing, and associated dose modifications/discontinuations.
The all-comers safety population included 398 patients in the talazoparib + enzalutamide arm, with a data cutoff of August 16, 2022. The median treatment duration of talazoparib was 19.8 months (range 0.0-42.8). All-cause any-grade treatment-emergent adverse events were observed in 98.5% of patients. The 3 most common nonhematologic treatment-emergent adverse events were fatigue (33.7%; 4.0% grade 3), back pain (22.1%; 2.5% grade 3), and decreased appetite (21.6%; 1.3% grade 3):

Treatment emergent adverse events occurred early in treatment and were generally manageable through dose modifications and supportive care. The incidence of most common hematologic grade 3/4 adverse events by week is shown as follows:
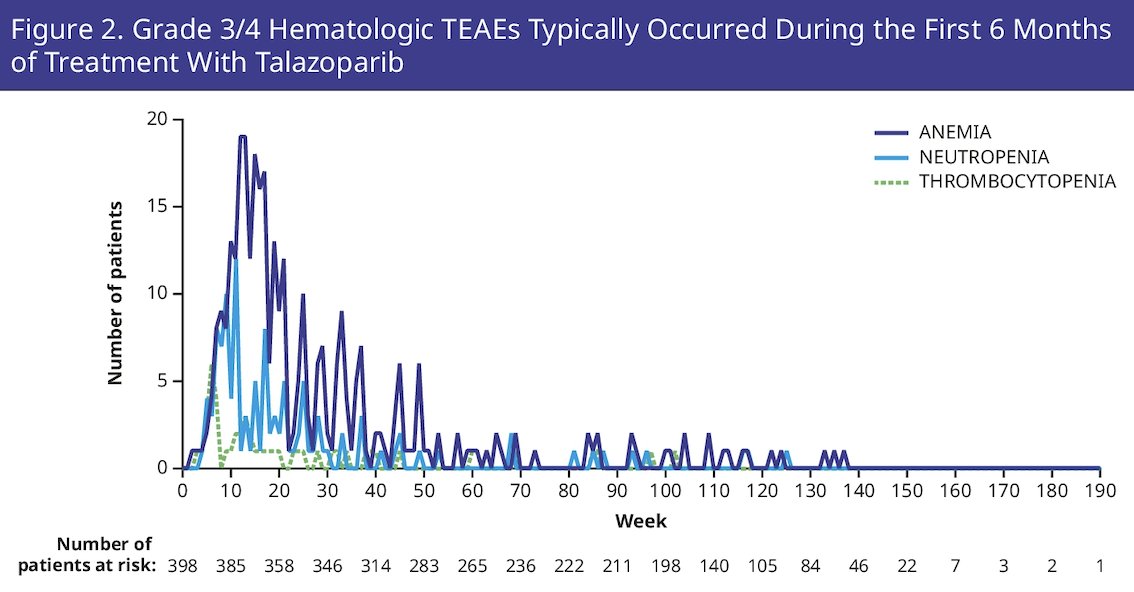
Overall, 19.1% of patients discontinued talazoparib due to treatment-emergent adverse events. To ensure optimal dosing of talazoparib at the individual level, the protocol did not require dose modification of talazoparib until anemia was grade ≥3, with a median time to onset of first grade ≥3 anemia of 3.3 months. The steepest reduction in hemoglobin levels was limited to the first 13 weeks of treatment:
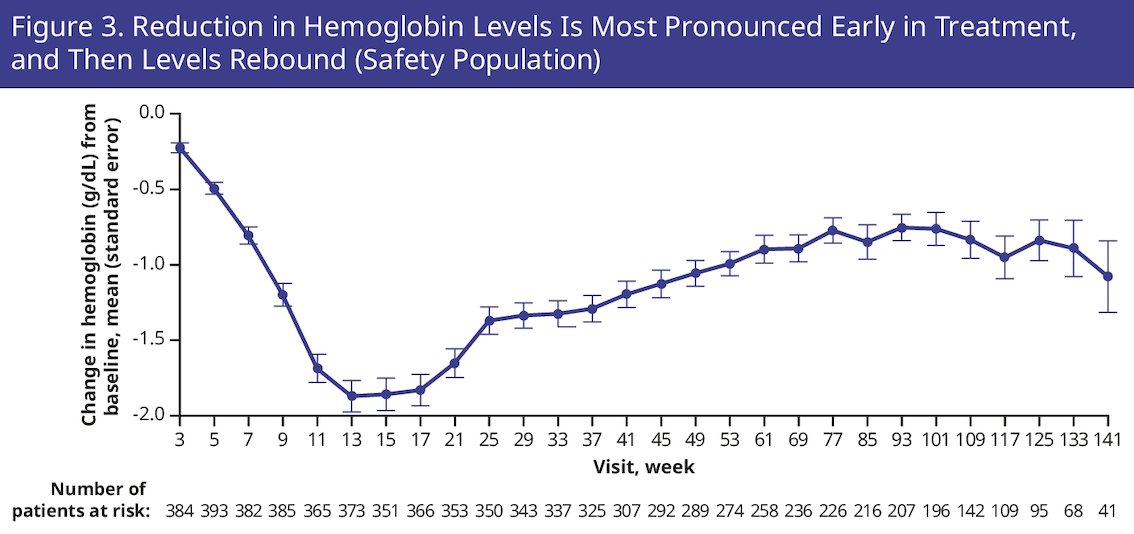
At study entry, 49.0% had grade 1–2 anemia. 43.2% had anemia leading to dose reduction (with or without transfusion) and 8.3% discontinued talazoparib due to anemia. A summary of supportive care measures for the treatment of anemia is as follows:
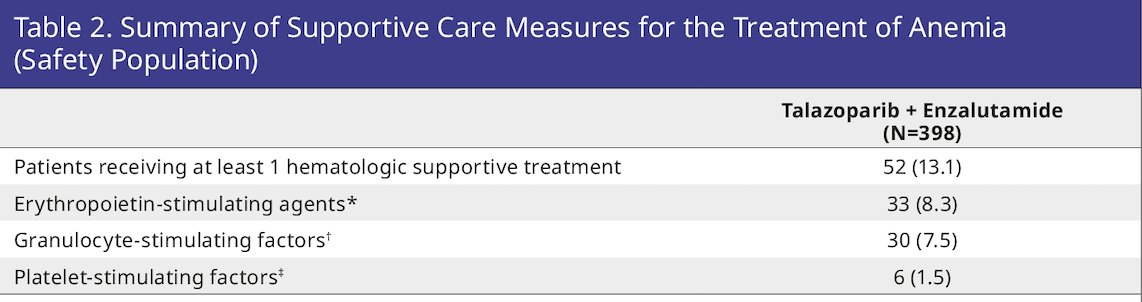
Despite dose reduction, the relative dose intensity of talazoparib remained >80% (approx. 0.4 mg/day).
Dr. Azad concluded his presentation by discussing safety analyses from TALAPRO-2 by highlighting the following take-home points:
- Talazoparib 0.5 mg QD + enzalutamide 160 mg QD was generally manageable, with dose modifications of talazoparib and/or standard supportive care
- Anemia was the most common treatment-emergent adverse event and led to discontinuation of talazoparib in 8.3% of patients
- Grade ≥3 hematologic adverse events typically occurred within 6 months of starting therapy and resolved in <1 month
- A detailed understanding of the onset, duration, and severity of adverse events, as well as the impact of dose adjustments in the TALAPRO-2 study, will optimize the management of patients receiving talazoparib + enzalutamide for mCRPC
Presented by: Arun Azad, MBBS, PhD, Peter MacCallum Cancer Centre, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet 4 June 2023 [Epub ahead of print].


