(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a session on prioritizing and sequencing therapy for advanced prostate cancer. Dr. Anis Hamid discussed the current treatment landscape of metastatic hormone-sensitive prostate cancer (mHSPC), debating against routine, early treatment intensification for mHSPC patients and arguing that ‘less is more’ in this scenario.
Dr. Hamid began by presenting the case of a 66-year-old male who underwent a radical prostatectomy in 2010 for Grade Group 3 disease, subsequently received salvage radiotherapy + a short course of ADT three years later in 2013, and then was started on doublet ADT+ abiraterone/prednisone 4 years later when he was found to have metachronous, CHAARTED low-volume, LATITUDE low-risk disease on PSMA PET imaging.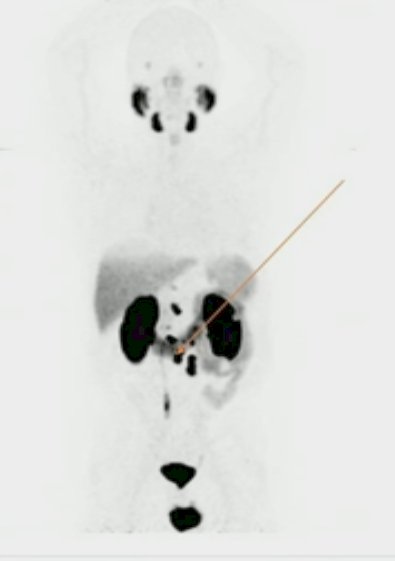
This patient subsequently had an excellent PSA response with a PSA of 0.05 ng/ml within 6 months of initiating treatment. 6 years later the patient remained on continuous therapy with undetectable PSA levels, but with mild-moderate side effects, including grade 1+ fatigue, tolerable hot flashes, easy bruising, and osteopenia on bone densitometry. Dr. Hamid asked: Is this patient a suitable candidate for treatment de-intensification in this setting?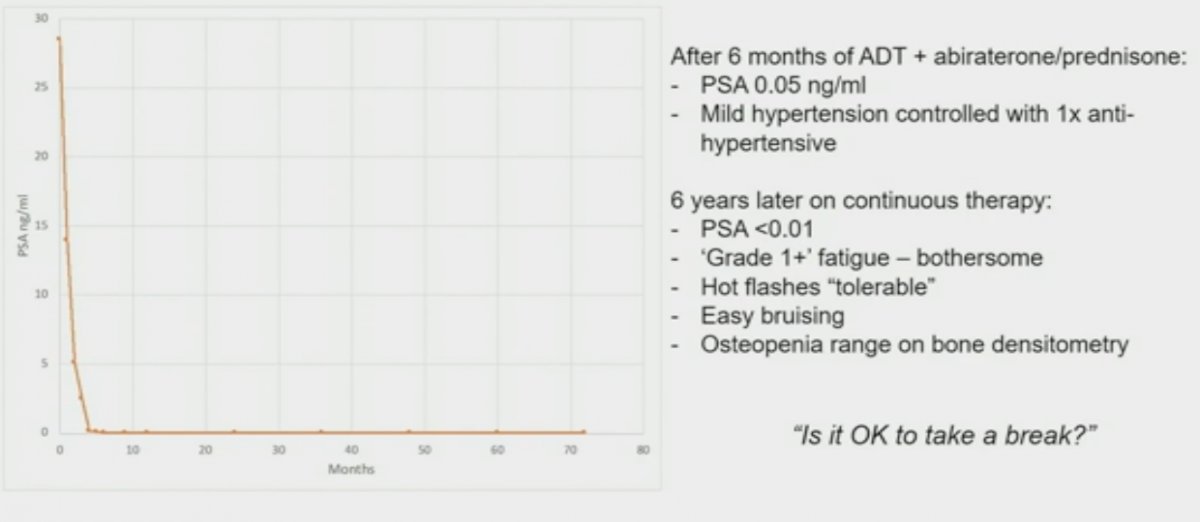
When considering systemic treatment options for mHSPC patients, it is important to consider the timing of disease presentation (synchronous versus metachronous) and the volume of disease (high versus low volume). Differences in clinical outcomes by disease volume and presentation/timing are reproducible across clinical trials and retrospective/registry datasets. When considering these two variables in tandem, four distinct mHSPC subgroups become clinically relevant (median overall survival [OS] per CHAARTED and GETUG-15):
- Metachronous and low volume: ~8 years
- Metachronous and high volume: 4.5 years
- Synchronous and low volume: 4.5 years
- Synchronous and high volume: 3 years
The recently published update of the ENZAMET trial clearly demonstrates this whereby patients with metachronous, low volume disease had significantly better 5-year cancer-specific survival rates (90%), compared to patients with synchronous, high-volume disease (58%). We also need to consider the competing risk of death from other causes, which occur in 44% of patients in the metachronous, low volume group within 5 years of randomization, compared to only 12% of patients in the higher risk group of synchronous, high-volume disease.1 These data suggest adopting less aggressive, risk-adapted approaches in these metachronous, low volume mHSPC patients, sparing them the toxicity of additive treatment intensification. Furthermore, we need to consider patients’ concerns in this setting. While the ASCO 2020 National Cancer Opinion Survey demonstrated that dying from cancer was the patients’ greatest concern regarding being diagnosed with cancer (62%), other significant concerns included the side effects of treatment (60%), long-term complications (42%), and the financial fears of financial impact on the family (34%) and paying for treatment (28%). This highlights the importance of considering other patient-relevant issues, beyond just OS outcomes, when considering treatment intensification.
Furthermore, we need to consider patients’ concerns in this setting. While the ASCO 2020 National Cancer Opinion Survey demonstrated that dying from cancer was the patients’ greatest concern regarding being diagnosed with cancer (62%), other significant concerns included the side effects of treatment (60%), long-term complications (42%), and the financial fears of financial impact on the family (34%) and paying for treatment (28%). This highlights the importance of considering other patient-relevant issues, beyond just OS outcomes, when considering treatment intensification.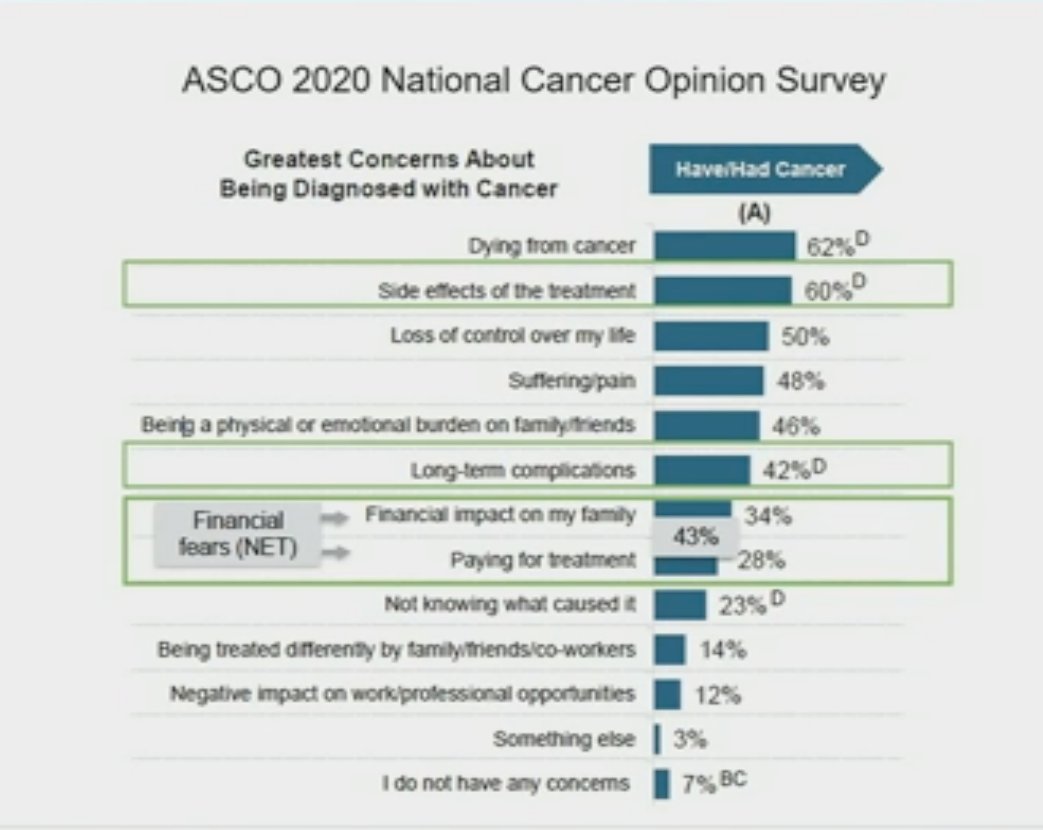
Patient-reported outcomes (PROs) from androgen receptor signaling inhibitor (ARSI) doublet therapy trials clearly demonstrated that ARSIs are associated with improvements in health-related quality of life (HRQoL) measures, time to pain progression, and other symptoms and functional domains, including in high-risk mHSPC populations. These benefits are most prominent early on with these treatments and likely reflect early enhanced disease control with treatment intensification.2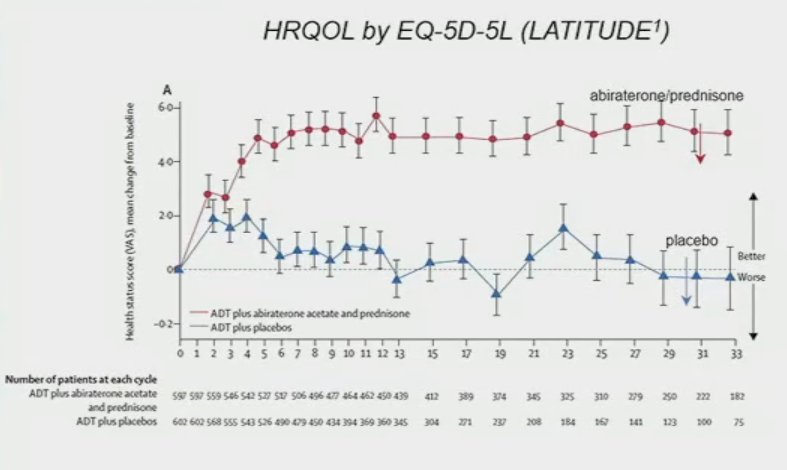 We also note an early decline in key symptom domains followed by stable differences thereafter, with cross-over in time to deterioration likely owing to improved disease control.1,3
We also note an early decline in key symptom domains followed by stable differences thereafter, with cross-over in time to deterioration likely owing to improved disease control.1,3
In addition to disease volume and timing/presentation, can we use other biomarkers to guide treatment (de)intensification approaches? The best characterized response-based endpoint that guides prognosis in mHSPC patients is the PSA nadir within 6 months of treatment initiation. Achieving a 6-month PSA nadir of <0.1 ng/ml has been consistently shown to be associated with improved survival endpoints across the major trials in the mHSPC disease space. As such, patients achieving this landmark may be suitable candidates for treatment de-intensification.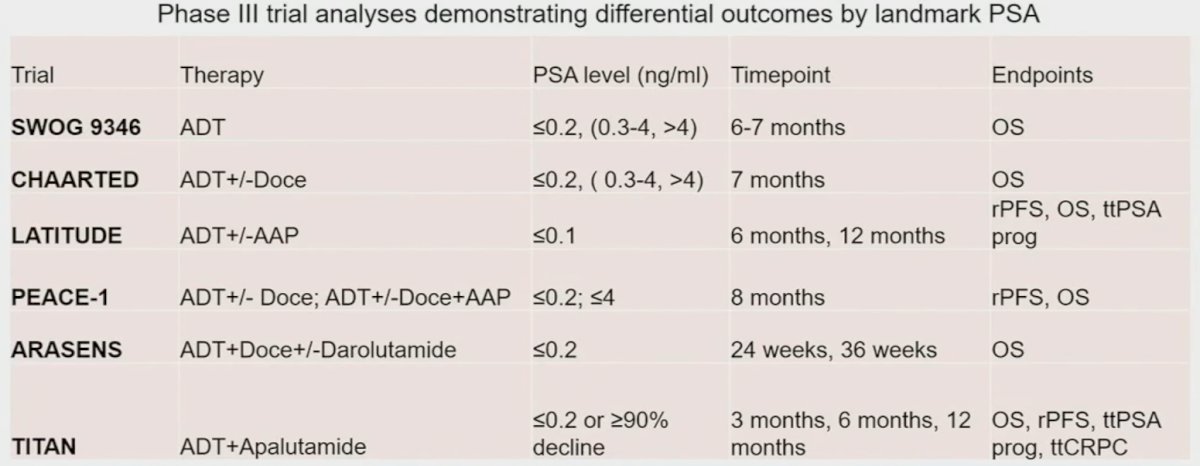
This is further highlighted by a recent report from the TITAN trial that demonstrated that early (3 months) and deep PSA responses with an ADT + an ARSI are associated with significantly prolonged OS (HR: 0.35, 95% CI: 0.25 – 0.48).4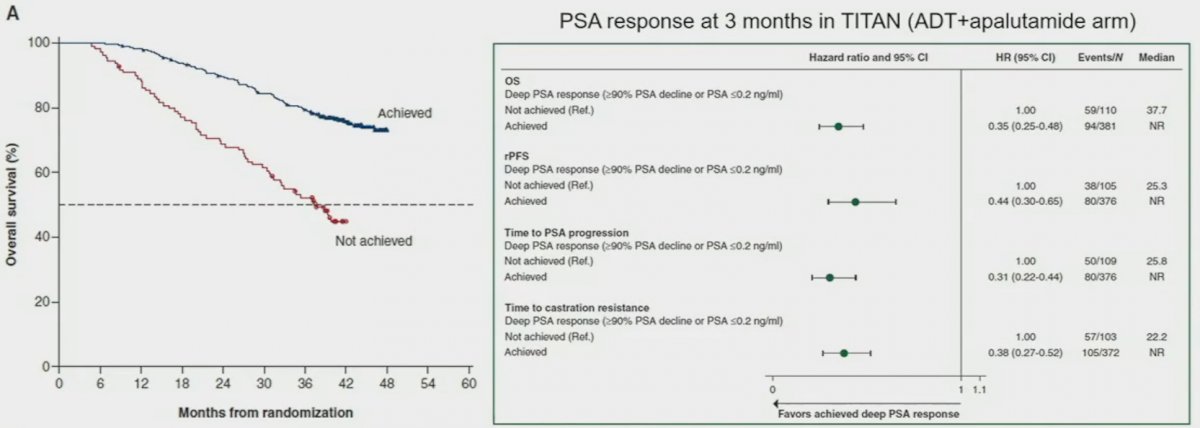
Although it is clear that early PSA response is a strong prognostic biomarker, there is still a paucity of high-level, prospective data to inform the decision for continuous versus intermittent treatment strategies in the era of combination systemic therapy. The SWOG 9346 trial by Dr. Hussain et al. randomized mHSPC patients with a PSA of 5 ng/ml or higher to receive combined ADT for 7 months following which patients with a PSA of 4 ng/ml or less were randomized to either continuous or intermittent ADT. This trial demonstrated the non-inferiority of intermittent ADT in this setting (HR for intermittent ADT: 1.10, 90% CI: 0.99 – 1.23), with benefits in the following quality of life domains: mental health, physical functioning, erectile function, and libido at 9 months favoring intermittent therapy.5 However, whether we can translate the results of this study to the doublet/triplet therapy setting remains to be determined.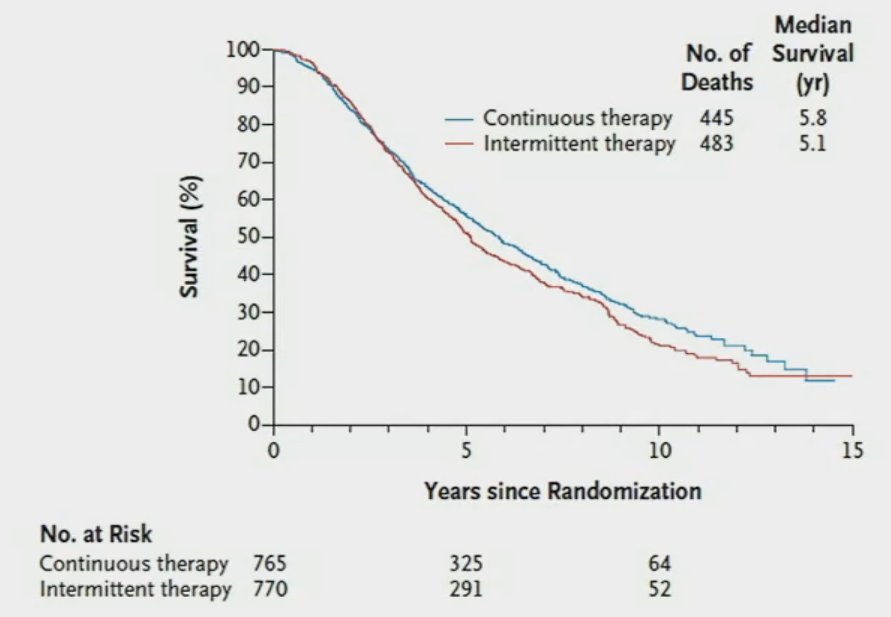
Currently, trials are underway attempting to address this clinical question. The A-DREAM trial is a phase 2 trial of ADT interruption in patients responding exceptionally to ARSIs in mHSPC. Patients with a PSA <0.2 ng/ml after 18-24 months of ADT and 12 months of an ARSI will be offered treatment interruption, with triggers for re-initiation including a PSA rise to 5 ng/ml or higher and/or development of radiographic changes or prostate cancer-related symptoms.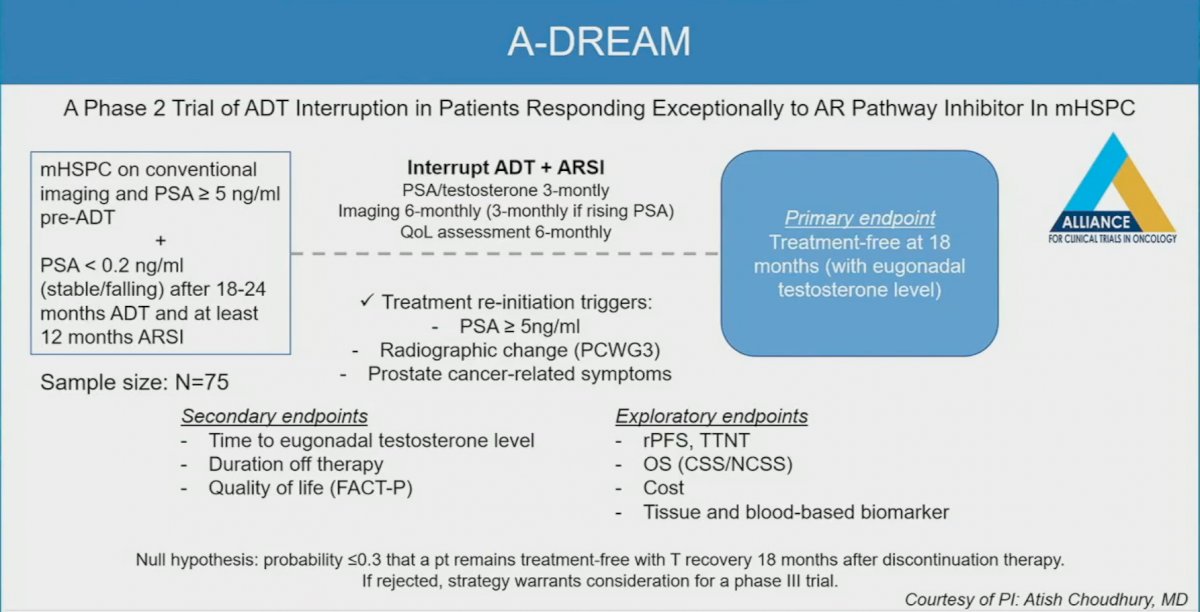
Similarly, the DE-ESCALATE trial, a phase III pragmatic trial, will evaluate intermittent ADT in the era of ARSIs, with treatment suspensions offered to patients who achieve a PSA of 0.2 ng/ml or less following 6-12 months of ADT + ARSI +/- docetaxel.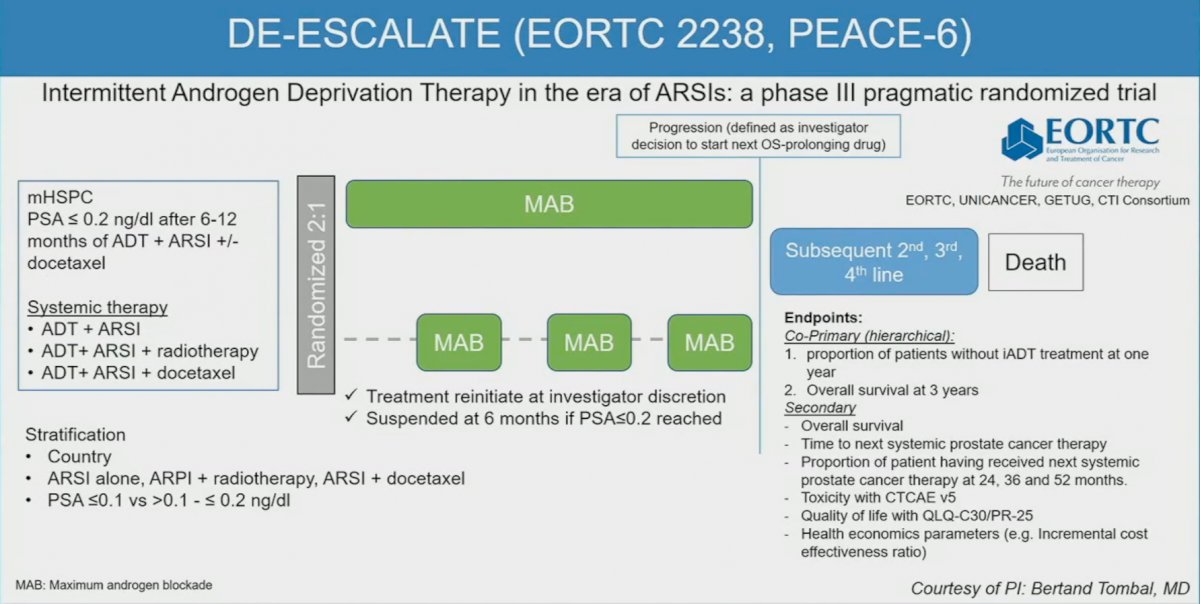
What about metastasis-directed therapy in the mHSPC disease setting. STOMP was a phase II trial of oligorecurrent mHSPC with 3 or less lesions on choline PET/CT. Patients randomized to MDT, in the form of SABR or surgery, had significantly prolonged ADT-free survival (21 versus 13 months).6 ORIOLE similarly evaluated MDT (SABR) in oligorecurrent mHSPC patients with 3 or less lesions on conventional imaging. Patients treated with MDT had significantly improved 6-month progression rates (61% versus 19%). This benefit was most pronounced in patients who had all PET-detected lesions targeted by MDT treatment plans.7 A pooled analysis of the two trials published in 2022 demonstrated that there was a proportion of these mHSPC patients (15-20%) treated with MDT who had PFS beyond 4 years. Notably, the median rPFS with MDT and in the no high-risk mutations cohort was 25.3 months.8
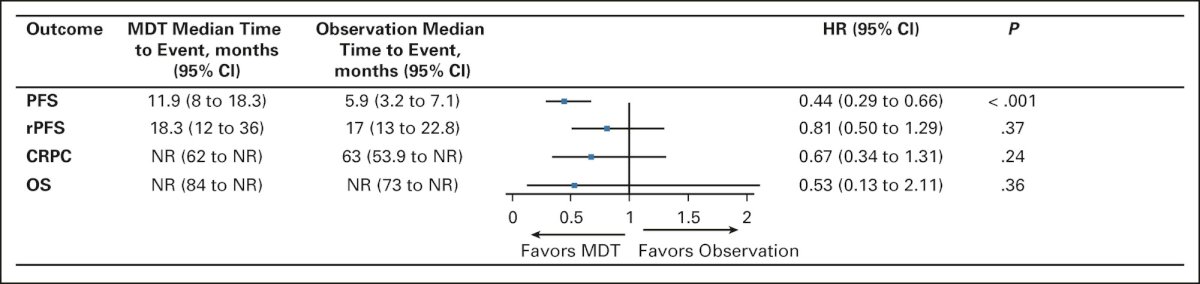
The recently published EXTEND trial evaluated the utility of adding MDT to intermittent hormonal therapy. This phase II trial randomized patients with oligometastatic hormone-sensitive or resistant prostate cancer (0-5 lesions) to six months of hormonal therapy followed by intermittent hormonal therapy with or without MDT. This trial met its primary endpoint with prolonged PFS in the MDT arm (HR: 0.25, p<0.001). Notably, a benefit was observed in the mHSPC subgroup (HR: 0.22), with consistent benefits across subgroups, including: synchronous versus metachronous and ADT + ARSI versus ADT alone.9 Significantly, eugonadal PFS (i.e., PFS in those who recovered normal testosterone levels following intermittent hormone therapy suspension) was significantly prolonged in the MDT group (HR: 0.32, 95% CI: 0.11 – 0.91, p=0.03).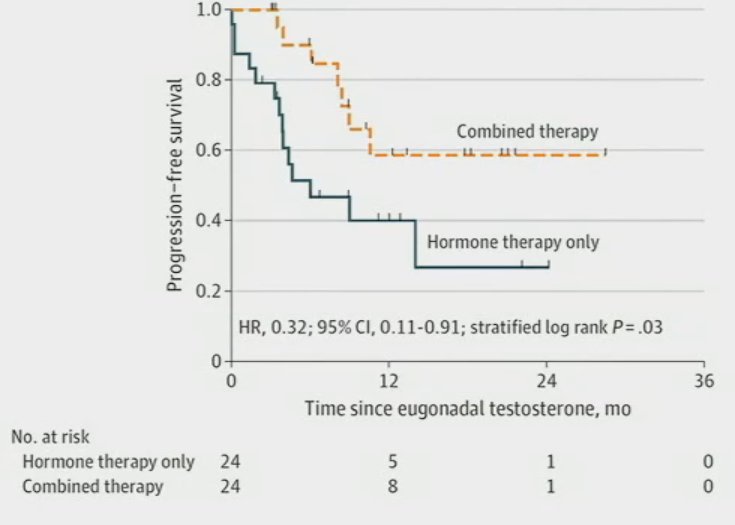
Non-castrating approaches are also being evaluated in select mHSPC populations with 177Lu-PSMA-617 recently evaluated as monotherapy for patients with metachronous, low-volume mHSPC disease with promising initial results.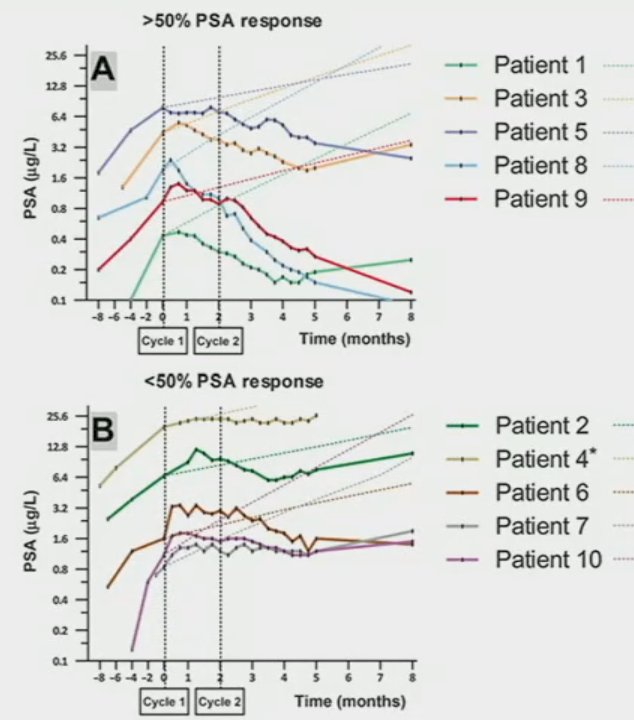
Other trials in this space include DART (SABR +/- 6 months of darolutamide) and RAVENS (SABR +/- Ra-223).
Dr. Hamid noted that other biomarkers in this setting, beyond PSA, are emerging and include lower tumor copy number alterations, which are associated with reduced risk of progression and death in the STAMPEDE ADT arm, and transcriptional subtypes (luminal-basal, AR activity, and Decipher® risk grouping) recently assessed within the CHAARTED ADT arm.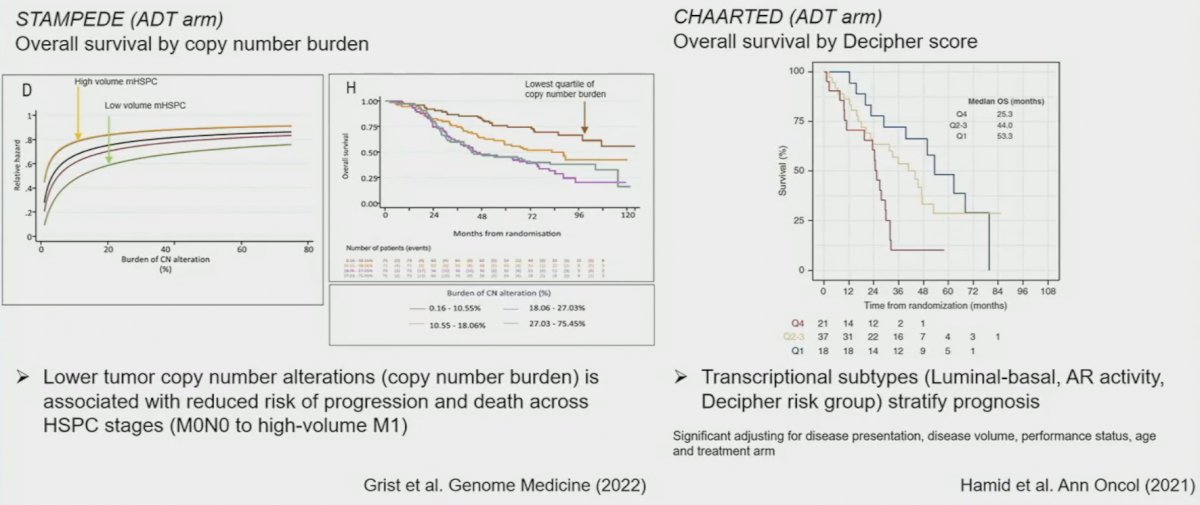
Dr. Hamid concluded his presentation with his thoughts on future directions in adaptive de-escalation in mHSPC.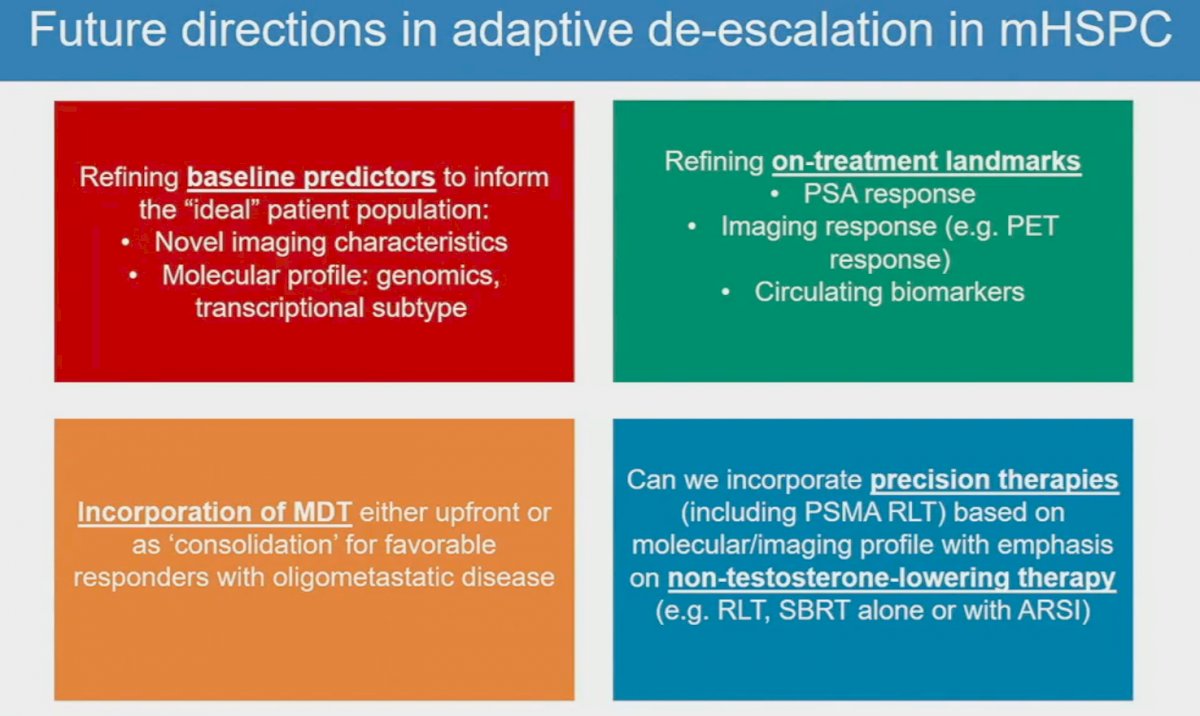
Presented by: Anis Hamid, MBBS, GU Oncology Research Fellow, Department of Medical Oncology, Peter MacCallum Cancer Centre and University of Melbourne, Melbourne, Victoria, Australia
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:- Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323-334.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: Final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294-2303.
- Chowdhury S, Bjartell A, Agarwal N, et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Ann Oncol. 2023;34(5):477-485.
- Hussain M, Tangren CM, Berry DL, et al. Intermittent versus Continuous Androgen Deprivation in Prostate Cancer. N Engl J Med. 2013;368:1314-1325.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol. 2018;36(5):446-53.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Deek MP, Van der Eecken K, Sutera P, et al. Long-term outcomes and genetic predictors of response to metastasis-directed therapy versus observation in oligometastatic prostate cancer: Analysis of STOMP and ORIOLE trials. J Clin Oncol. 2022;40(29):3377-3382.
- Tang C, Sherry AD, Haymaker C, et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023.


