(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers poster session. Dr. Dong-Woo Kang presented the rationale and study framework for the POWER trial, which aims to evaluate exercise to enhance cardiovascular health among Black patients with prostate cancer treated with androgen deprivation therapy (ADT).
Black patients with prostate cancer have a 1.8-fold increased hazard of being diagnosed with prostate cancer, and a 2.2-fold increased rate of prostate cancer-related death when compared to non-Hispanic, White prostate cancer patients.1 Furthermore, black patients treated with ADT also have an increased risk of cardiovascular disease, which is likely secondary to the adverse effects of ADT on individual cardiovascular risk profile domains, including weight gain, worsening of serum lipid profile, decreased lean mass, and worsened endothelial cell function. One potential solution is exercise, which has been consistently demonstrated to be a safe and effective intervention for counteracting these adverse effects. Significantly, though, Black patients are disproportionately under-represented in prior studies, and the feasibility/efficacy of exercise in these studies, mainly including White men, cannot necessarily be generalized to Black prostate cancer patients due to known sociodemographic differences. As such, the objective of the POWER trial (Exercise to Enhance Cardiovascular Health among Black Prostate Cancer Patients with Androgen Deprivation Therapy; NCT05327465) is to assess the impact of exercise, compared to usual care, on the cardiovascular risk profile domains in Black men with prostate cancer receiving ADT.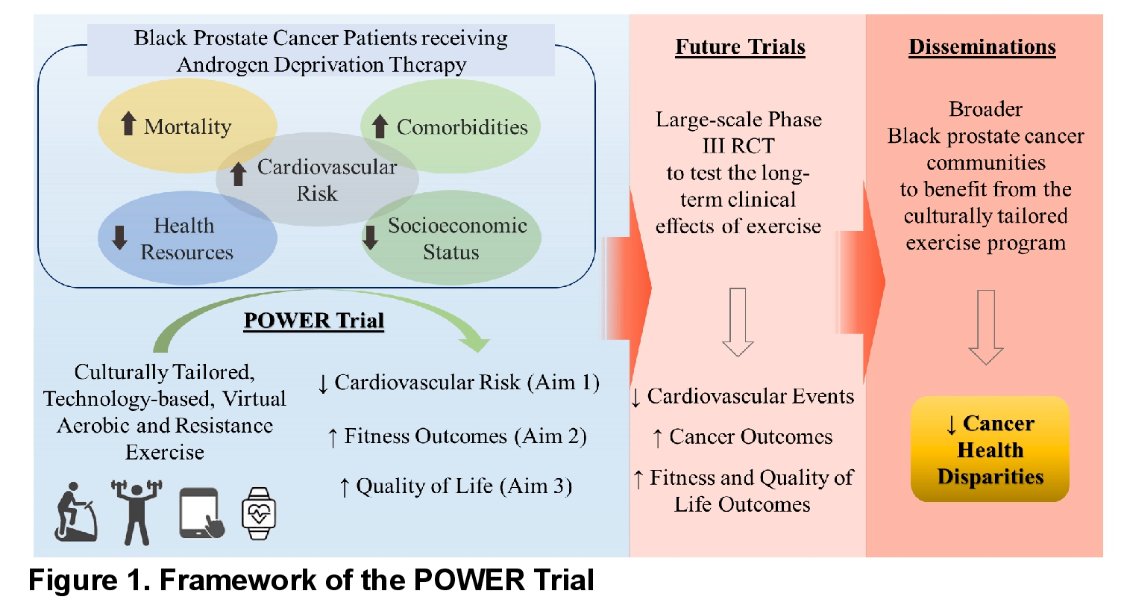
POWER is a multicenter, two-armed, phase II randomized clinical trial currently enrolling at the Dana-Farber Cancer Institute and the Beth Israel Deaconess Medical Center in Boston, MA. This trial will randomize 62 patients 1:1 to either exercise or usual care for 16 weeks.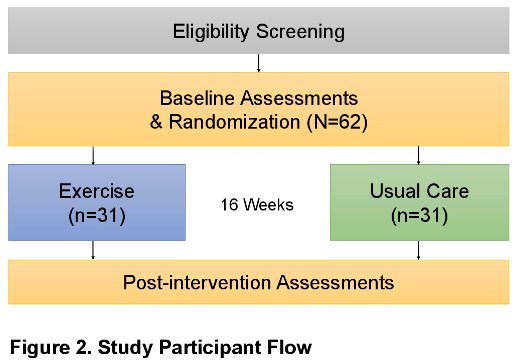
Study eligibility criteria are as follows:
- Black men ages 18+ years with prostate cancer
- Actively receiving ADT for >4 months at the time of recruitment
- Clinician clearance for exercise participation
- Physically inactive, defined as less than 60 minutes of structured exercise per week
- English- or Spanish-speaking
- Patients with any uncontrolled comorbidity or contraindication that may be exacerbated during exercise will be excluded

Patients randomized to the intervention arm will be instructed to exercise three times per week for 16 weeks. A virtual intervention conduce will be delivered via Zoom. Patients will be provided home-exercise equipment at no cost and will be supervised by a certified clinical exercise physiologist. Patients will gradually “ramp up” the exercise duration and intensity with sessions planned for 60 to 90 minutes. Patients will undergo both aerobic and resistance exercises. A sociocultural training component will be adapted as follows:
- Accessible virtually supervised, home-based exercise
- Bi-weekly newsletters
- Monthly group-based virtual exercise sessions
- Individual orientation session
- Black prostate cancer support groups
Patients in the usual care group will be asked to maintain their baseline behavior for 16 weeks and will be subsequently offered the exercise intervention components after the initial 16-week study period.
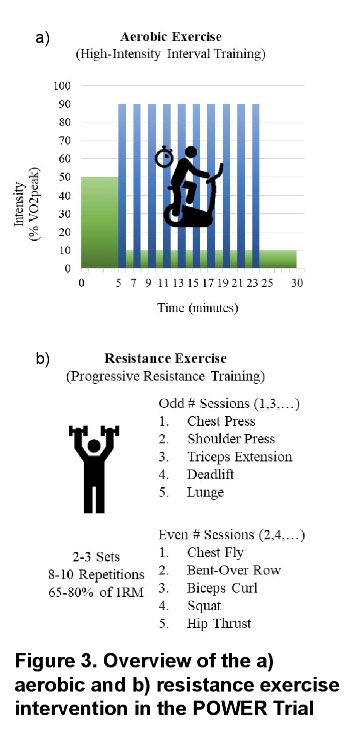
The primary outcome is cardiovascular risk assessed via the Framingham Risk Score (FRS). Secondary outcomes will include:
- Cardiovascular capacity (peak oxygen consumption)
- Physical fitness and function
- Body composition (DXA)
- Treatment symptoms
- Quality of life
- Intervention feasibility and acceptability
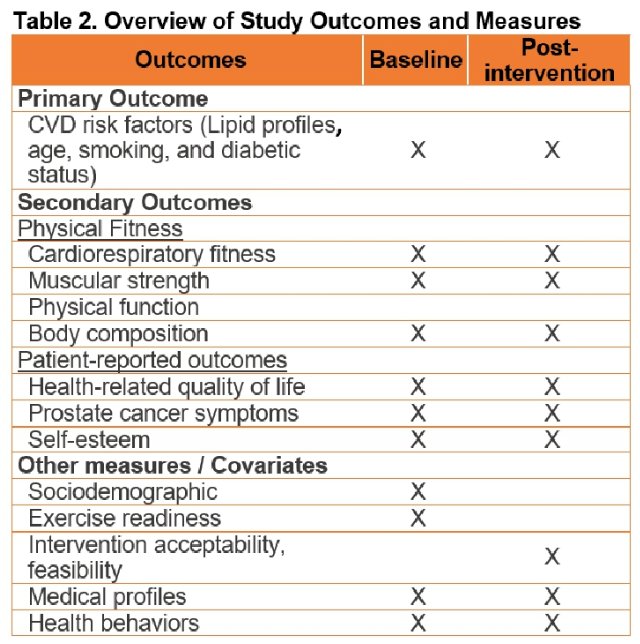
Given the repeated nature of the measurements within the same individuals, the ANCOVA model will be used to evaluate for between-group differences, adjusted for baseline covariate that are potentially associated with FRS changes, including baseline FRS, history of cardiovascular disease, use of medication for hypertension and/or hyperlipidemia.
At the time of abstract submission, five out of the planned 62 patients had been enrolled.Presented by: Dong-Woo Kang PhD, Instructor, Division of Population Science, Department of Medical Oncology, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
Reference:- Dess RT, Hartman HE, Mahal BA, et al. Association of Black Race With Prostate Cancer–Specific and Other-Cause Mortality. JAMA Oncol 2019;5(7):975-983.


