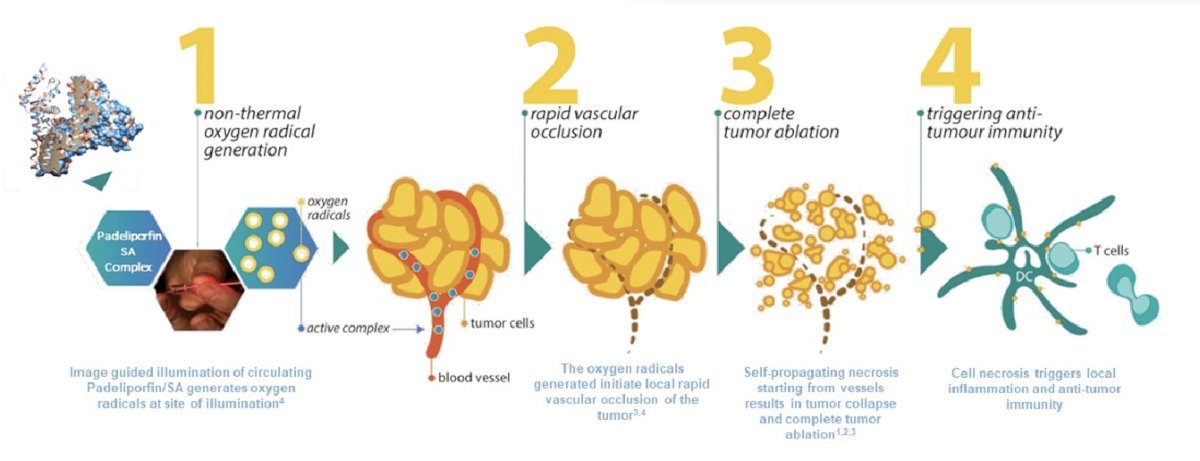
(UroToday.com) The 2023 ASCO annual meeting included a urothelial carcinoma session, featuring a trials in progress presentation by Evgenia Alpert discussing a phase 3 multicenter study to evaluate the safety and efficacy of padeliporfin vascular targeted photodynamic (VTP) therapy in the treatment of low-grade upper tract urothelial cancer (UTUC). Padeliporfin VTP is a combination product of drug therapy (padeliporfin is administered IV) and a device (laser light delivery system), which includes a source of light (the laser) that emits near-infrared (NIR) light at 753 nm, and optic fiber that delivers the light to the target lesion(s) in the upper tract urothelium:
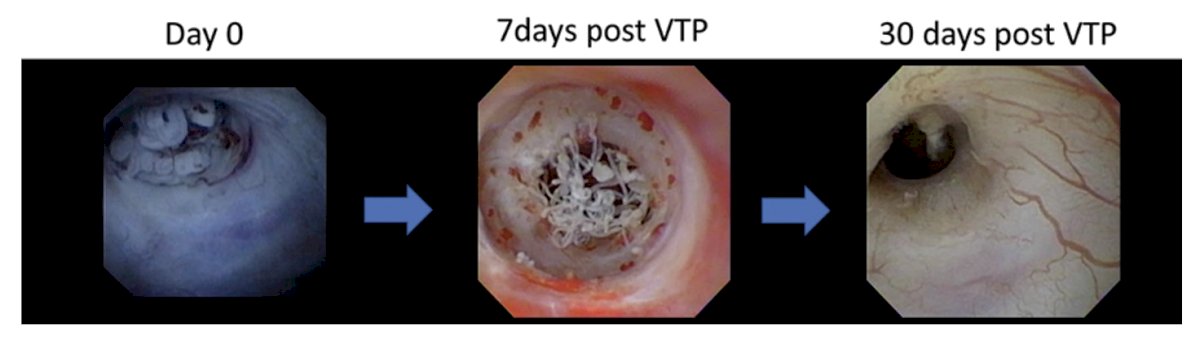
The following demonstrates the endoscopic appearance of the tumor before and after a single VTP treatment:
Padeliporfin VTP is being evaluated in a phase 3 study in patients with low grade UTUC, and 100 patients are planned to be enrolled. Enrollment started in March 2021 and is ongoing in USA, European Union and Israel. As of April 25, 2023, 3 patients have been enrolled and 5 were treated. Patients are being treated with padeliporfin VTP in two phases:
- Induction and maintenance treatment phases. Induction treatment phase consists of 1-3 VTPs every 28 days. If complete response is not achieved after 1st VTP, up to 2 additional VTPs are allowed. If complete response is achieved in the induction treatment phase, patients are allowed into the maintenance treatment phase (12 months). In the maintenance treatment phase, VTPs can be provided every 3 months for patients with recurrent tumor that is deemed treatable, defined as low grade with the largest tumor (index) 5-15 mm, in up to 2 locations in the kidney or one ureter location ≤20 mm
- Long term follow-up phase: patients completing the maintenance treatment phase, will be followed for safety for 48 months with no VTPs. The primary objective is to demonstrate efficacy and durability of effect of padeliporfin VTP treatment. The secondary objective is evaluation of padeliporfin VTP‐related safety and tolerability
The trial design for ENLIGHTED is as follows:

Key inclusion criteria are (i) up to two biopsy-proven low grade UTUC tumors with the largest tumor (index) 5-15 mm, located in the calyces, renal pelvis, or in the ureter, with no high grade cells, and (ii) ureter involvement in one location ≤ 20 mm. Key exclusion criteria include (i) current high-grade or muscle invasive urothelial bladder cancer, (ii) current CIS or previous CIS in the upper urinary tract, and (iii) a history of ≥ T2 urothelial cancer in the past 2 years.
Evgenia Alpert concluded this presentation discussing ENLIGHTED by highlighting that the DSMB last reviewed the trial in April 2023 and suggested the trial continue as planned.
Presented by: Evgenia Alpert, Steba Biotech, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


