(UroToday.com) Dr. Cora Sternberg presents the final results of SAUL, a single-arm international real-world study of atezolizumab (atezo) in 1004 patients (pts) with pretreated locally advanced/metastatic urinary tract carcinoma (UTC).
In the SAUL study (NCT02928406),1 clinical outcomes in the general population were similar to findings from phase 3 trials of anti-PD-(L)1 despite opening up the inclusion to many patients who would have not been eligible for clinical trials. These types of trials are important, as many patients in the real-world setting do not fit the very strict inclusion criteria of clinical trials.
In this presentation, Dr. Sternberg report the final analysis 4 years after enrolling the last patient.
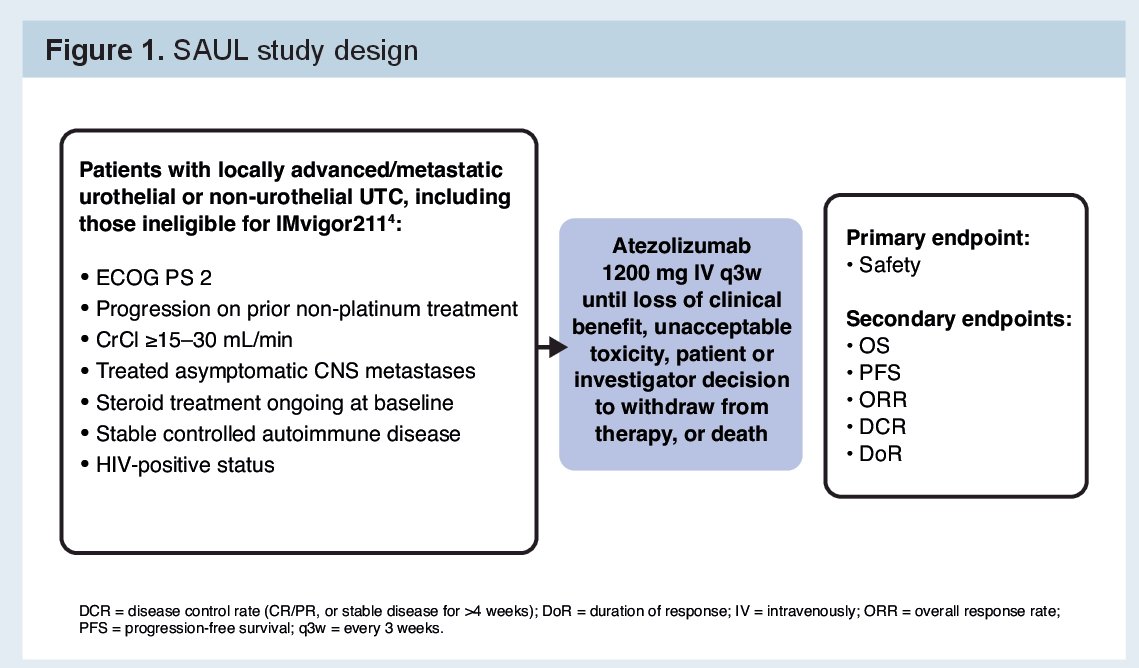
Eligible patients for this trial were those with locally advanced or metastatic urothelial or non-urothelial carcinoma that had progressed during or after 1–3 prior lines of therapy for advanced UTC (or within 12 months of [neo]adjuvant therapy). Unlike most phase 3 immunotherapy trials, they allowed patients with autoimmune disease (AID), ECOG PS 2, creatinine clearance (CrCl) ≥15–30 mL/min, and/or stable CNS metastases were eligible.
As per the medication approval, atezolizumab was given until disease progression or unacceptable toxicity. The primary endpoint was safety. Secondary endpoints included overall survival (OS) and duration of response (DoR).
SAUL ultimately enrolled 1004 patients from 172 sites in 32 countries.
Of these, 997 patients were treated.
At data cutoff (Dec 20, 2022), patients had a median follow-up of 55 month. 78% of patients had died. Median treatment duration was 2.8 months – of these patients, 96 pts (10%) took atezo for >3 years and 68 (7%) for >4 years. The most common reason for discontinuation was disease progression (75%).
Baseline characteristics of the overall cohort and special subsets (long responders) are seen below:
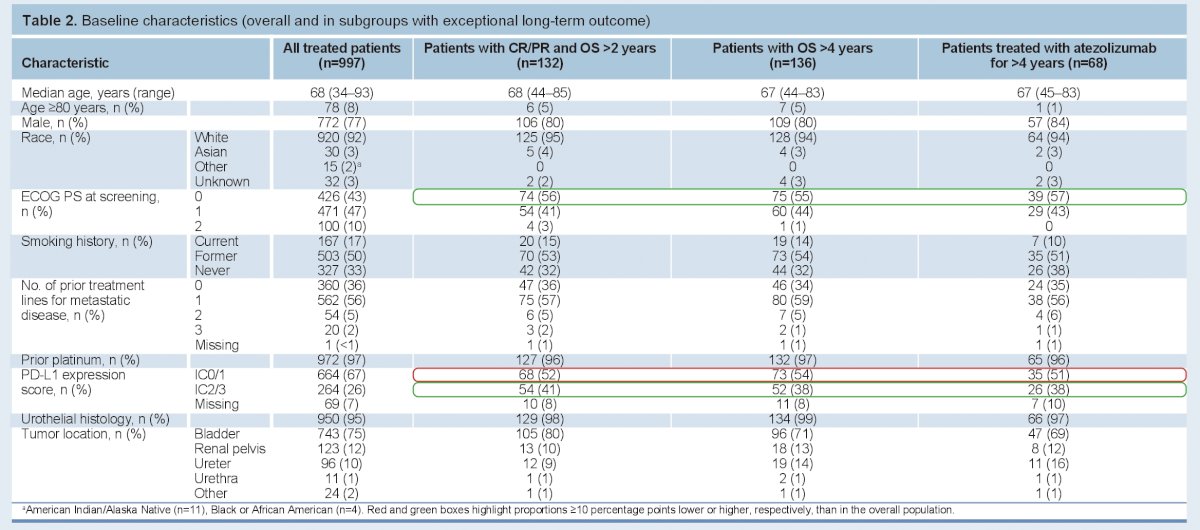
- Of note, 10% had ECOG PS 2, 5% had CrCl <30 mL/min, 4% had a history of autoimmune deficiencies, and 7% had 2–3 prior lines of therapy.
Safety:
Grade ≥3 adverse events (AEs) occurred in 51%. These led to atezo discontinuation in 8% of patients.
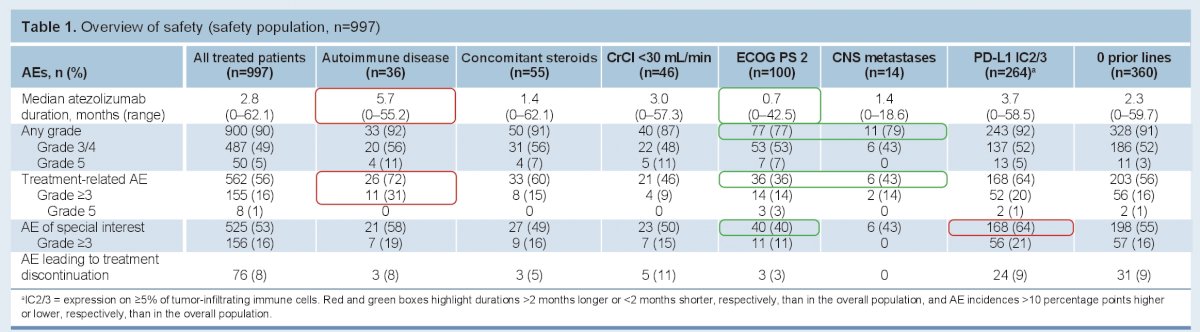
Importantly, it was well tolerated in many of the special populations, including patients who would have been ineligible for clinical trials. This includes patients with autoimmune diseases.
Efficacy:
As of December 2022, 78% of patients had died.
In the ITT population:
- median overall survival was 8.6 months
- ORR was 16%
- Median DoR was 27.8 months
- OS was >4 y in 136 pts (14%); 132 responders had OS >2 y.

Outcomes in overall and in key subgroups are summarized below:

Special groups had similar objective response rates to the entire cohort – but had significantly lower duration of response. Patients with higher PDL-1 and 0 prior lines of therapy did better in that regard.
Overall survival in the special interest groups is seen below:

After 55 months median follow-up, median OS was 8.6 mo and 3-y OS was 21% in a real-world population including important understudied subgroups. Among 136 patients with OS > 4 years, median treatment duration was 47.8 months. Long-term safety data demonstrated the generally well tolerated nature of atezolizumab, even in the real world setting and patients with complex comorbidities. Clinical outcomes were similar to the larger Phase 3 trials, despite the inclusion of these unselected patients. Ongoing analyses are attempting to characterize the patients with exceptional long-term outcomes.
As mentioned earlier, clinical trial inclusion criteria can be very restrictive. These real world studies provide a better perspective on the tolerability of these medications in the larger population.
Clinical trial information: NCT02928406.
Presented by: Cora Sternberg, MD, FACP Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:

