(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a kidney and bladder rapid oral abstract session. Dr. Jeremy Blanc presented the survival analysis of the AURA trial evaluating avelumab as neoadjuvant therapy in patients with muscle-invasive urothelial carcinoma.
Cisplatin-based neoadjuvant chemotherapy followed by surgery is the standard of care approach for patients with clinically localized (cT2-4aN0M0) muscle-invasive urothelial carcinoma of the bladder. However, it is estimated that nearly half of such patients are unfit for cisplatin-based chemotherapy with no alternative systemic therapy currently approved in the neoadjuvant setting. Neoadjuvant studies investigating immune checkpoint inhibitors (ICI) have demonstrated promising anti-tumor activity and survival outcomes:
- ICI single agent : pCR= 29–46%, >85% overall survival at three years1-3
- ICI plus chemotherapy : pCR= 33–43%, >81% overall survival at three years4-5
AURA is a multi-center, non-comparative randomized phase II trial investigating neoadjuvant avelumab alone or in combination with chemotherapy. Patients with muscle-invasive urothelial carcinoma of the bladder planned for a radical cystectomy + pelvic lymphadenectomy were randomized by cisplatin eligibility to neoadjuvant therapy as follows:
- Cisplatin-eligible (Cohort 1):
- Gemcitabine/cisplatin + avelumab
- Dose-dense MVAC + avelumab
- Cisplatin-ineligible + avelumab:
- Paclitaxel/gemcitabine + avelumab
- Avelumab monotherapy
The primary endpoint is the proportion of patients achieving a complete pathologic response (i.e., ypT0/TisN0). Secondary endpoints include:
- Proportion of patients achieving <ypT2N0
- Safety (CTCAE v4)
- Event-free survival (EFS) and overall survival at 12 and 36 months
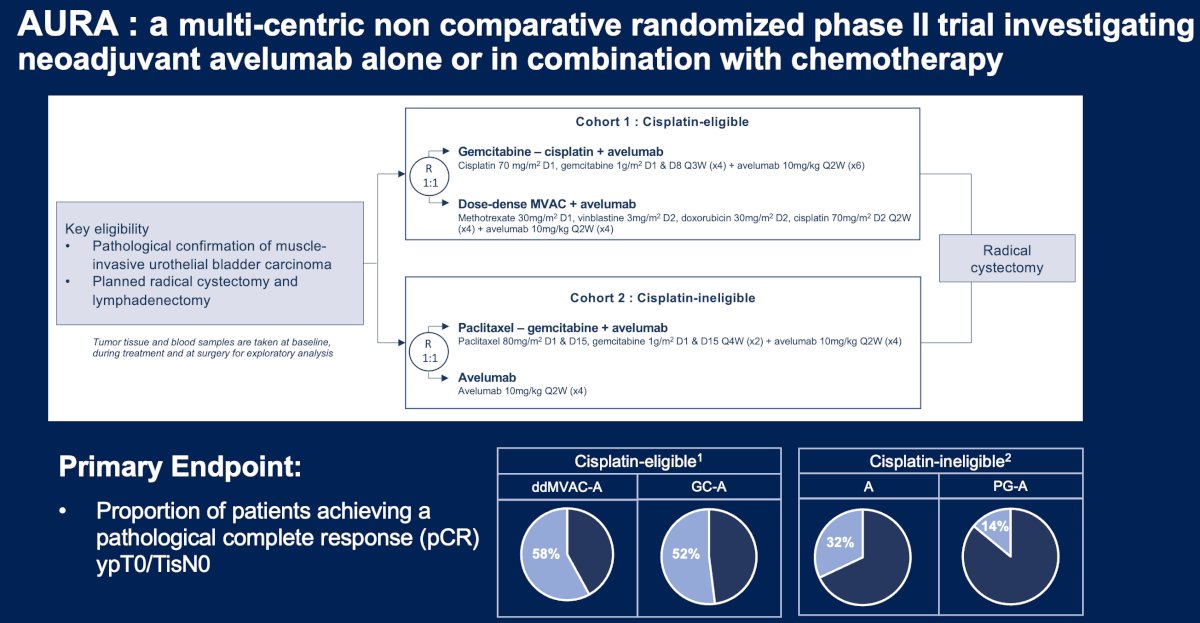
In the cisplatin-eligible cohort of patients, a pathologic complete response was observed in 22/38 (58%) patients in the ddMVAC + avelumab arm and 19/35 (54%) patients in the gemcitabine/cisplatin + avelumab arm. The 12-month EFS rates were 92% in the ddMVAC + avelumab arm, compared to 84% for gemcitabine/cisplatin + avelumab. The preliminary 36-month EFS rates were 79% and 62%, respectively. For overall survival, the 12 months rates were slightly higher in favor of ddMVAC + avelumab (95% versus 92%) and considerably higher at 36 months in favor of ddMVAC + avelumab (85% versus 64%).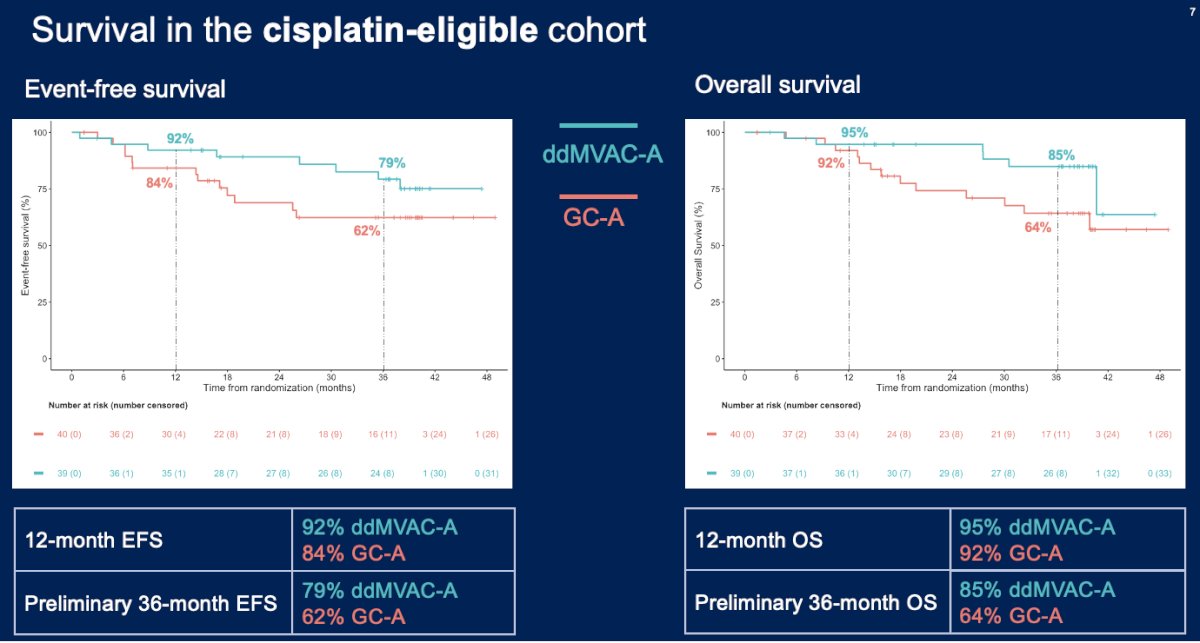
Patients with a pathologic complete response experienced significantly better overall survival outcomes, confirming the prognostic significance of this pathologic endpoint.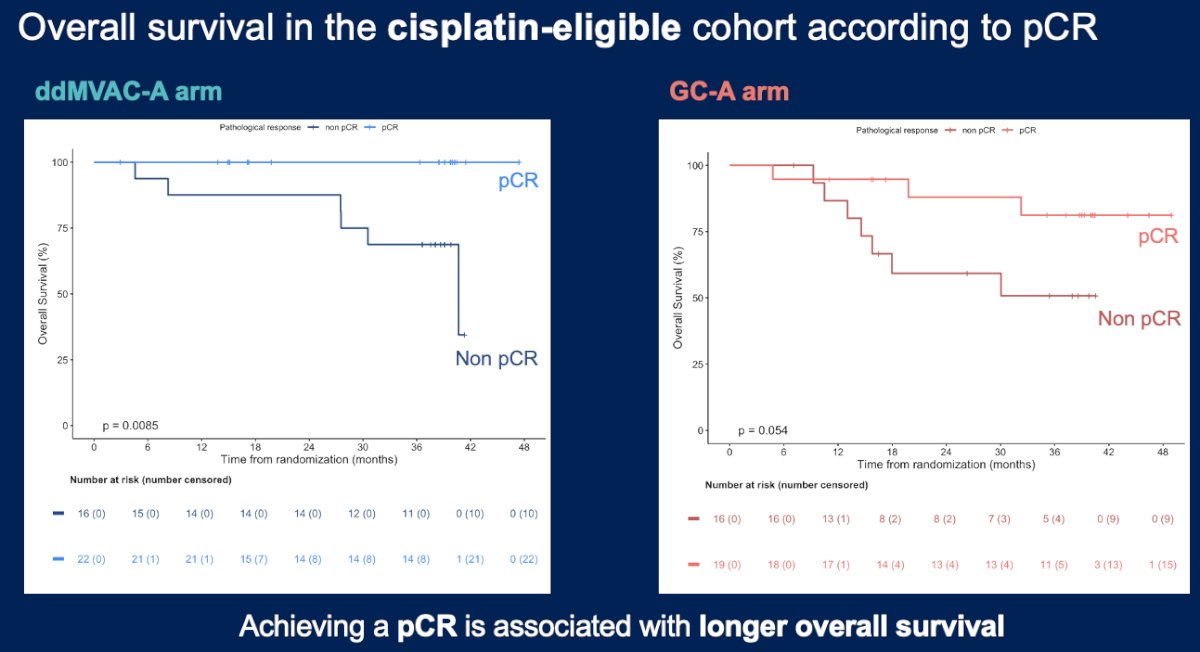
In the cisplatin-ineligible cohort, a pathologic complete response was observed in 4/28 (14%) and 9/27 (33%) patients in the paclitaxel/gemcitabine + avelumab and avelumab monotherapy arms, respectively. The event-free and overall survival rates were similar between the two arms: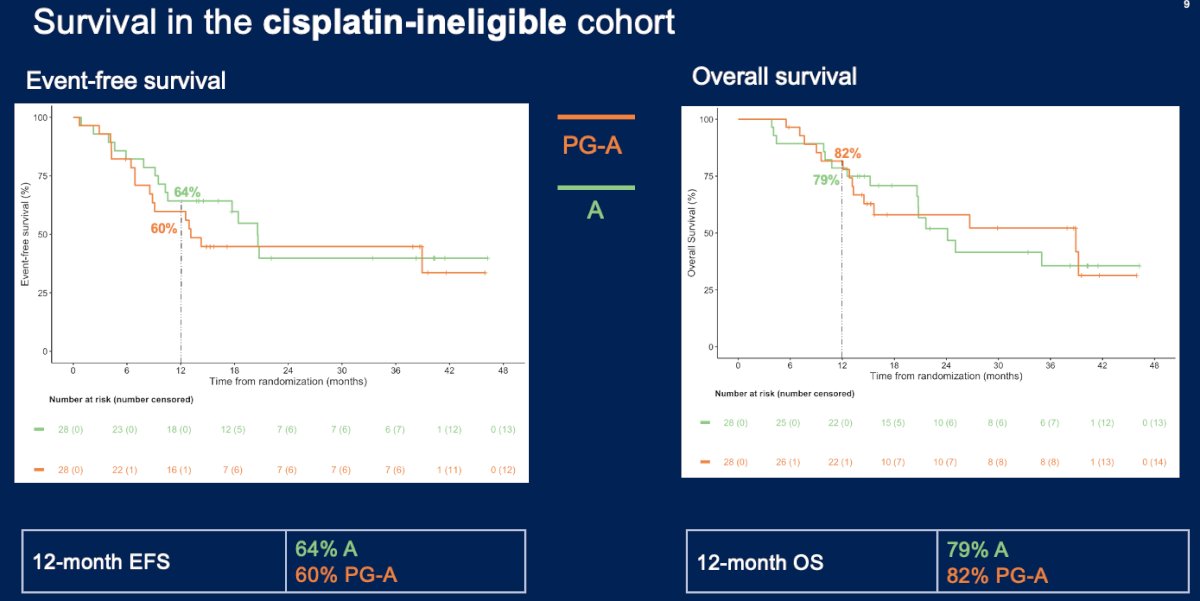
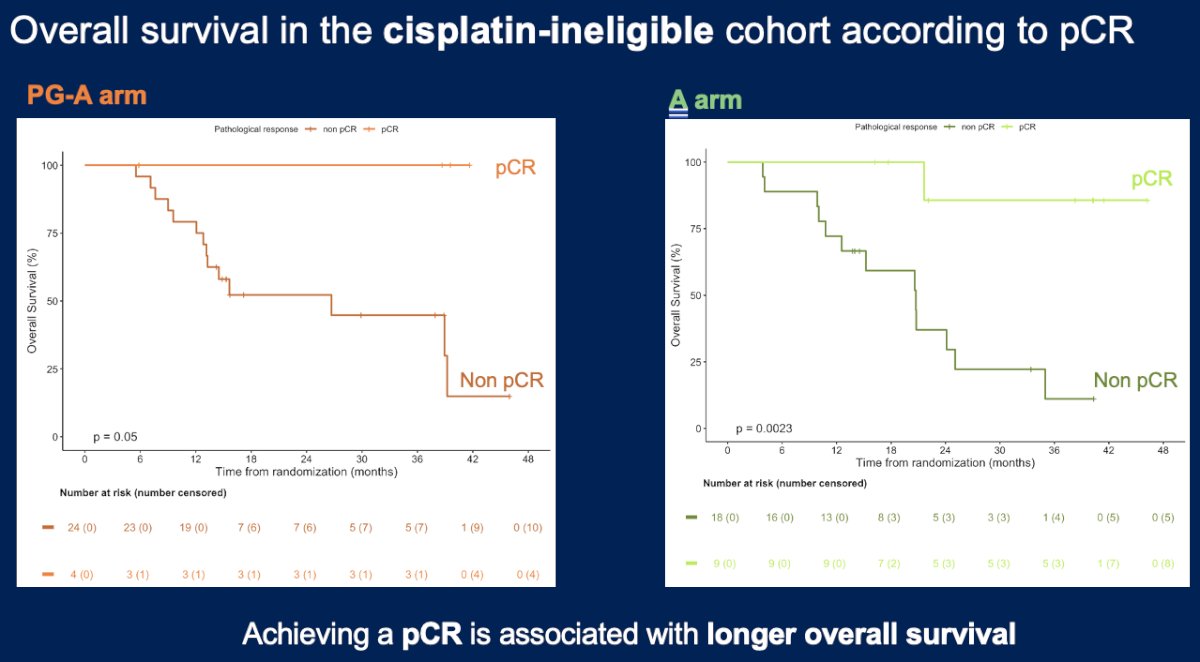
Dr. Blanc concluded as follows:
- Cisplatin-eligible cohort :
- High event-free and overall survival rates are achieved at 12 months and 36 months in patients treated with neoadjuvant avelumab in combination with cisplatin-based chemotherapies, especially in patients treated with ddMVAC + avelumab.
- Cisplatin-ineligible cohort :
- Lower survival outcomes are achieved at 12 months, with no additional benefit from addition of avelumab to paclitaxel/gemcitabine.
- Longer follow-up is needed for a 36-month survival analysis.
- Achieving a complete pathologic response is correlated with better survival outcomes for each treatment arm.
- Further investigation through phase III trials is essential to validate these findings including biomarker identification for optimizing muscle-invasive bladder cancer care and patient selection.
Presented By: Jeremy Blanc, MD, Institut Jules Bordet, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles, Brussels, Belgium
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:- Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2018;36(34):3353-60.
- Basile G, Banidin M, Gibb EA, et al. Neoadjuvant Pembrolizumab and Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Cancer: 3-Year Median Follow-Up Update of PURE-01 Trial. Clin Cancer Res 2022;28(23):5107-14.
- Powles T, Kockx M, Rodriguez-Vida, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med 2019; 25(11):1706-14.
- Funt SA, Lattanzi M, Whiting K, et al. Neoadjuvant Atezolizumab With Gemcitabine and Cisplatin in Patients With Muscle-Invasive Bladder Cancer: A Multicenter, Single-Arm, Phase II Trial. J Clin Oncol. 2022;40(12): 1312-22.
- Cathomas R, Rothschild SI, Hayoz S, et al. Perioperative Chemoimmunotherapy With Durvalumab for Muscle-Invasive Urothelial Carcinoma: Primary Analysis of the Single-Arm Phase II Trial SAKK 06/17. J Clin Oncol. 2023;41(33): 5131-9.


