(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a kidney and bladder rapid oral abstract session. Dr. Antonio Cigliola presented the interim results of SURE-01/02 evaluating perioperative sacituzumab govitecan alone or in combination with pembrolizumab for patients with muscle-invasive urothelial carcinoma of the bladder (MIBC).
The prognosis of cisplatin-ineligible patients with MIBC remains poor in up to 50%, and upfront radical cystectomy or chemoradiation is the current standard of care approach. Sacituzumab govitecan (SG) has gained US FDA-accelerated approval for the treatment of locally advanced or metastatic urothelial carcinoma (UC) based on the results of TROPHY-U-01.1,2
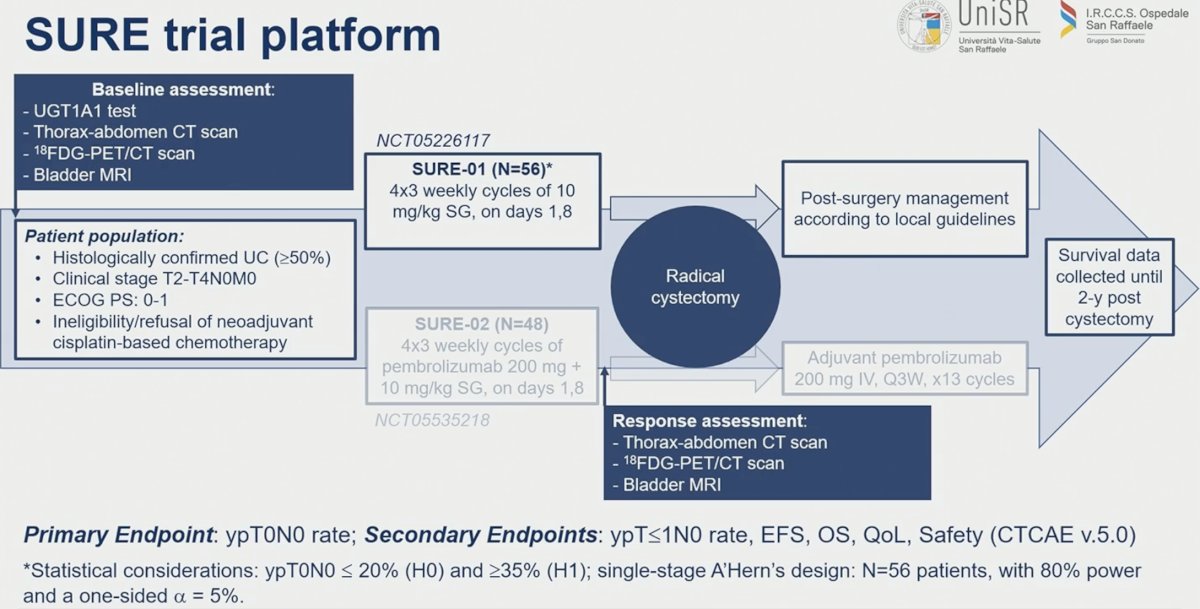
SURE is a multi-cohort, open-label, phase 2 trial evaluating neoadjuvant SG in MIBC patients either as:
- Monotherapy (SURE-01 trial, NCT05226117)
- In combination with pembrolizumab, followed by postsurgical adjuvant pembrolizumab (SURE-02 trial, NCT05535218)
In this study, Dr. Cigliola reported the interim results from SURE-01. The SURE trial platform is illustrated below. In SURE-01, patients with histologically confirmed urothelial carcinoma (≥50% component) with cT2-4N0M0 disease and either ineligible for or refused neoadjuvant cisplatin-based chemotherapy received four 3-weekly cycles of SG at a dose of 10 mg/Kg on days 1 and 8 and subsequently underwent a radical cystectomy. Prior to surgery, patients underwent a response assessment using a CT scan of the abdomen and chest, an 18FDG-PET/CT, and a bladder MRI. The primary endpoint was ypT0N0 rate. Secondary endpoints included:
- ypT≤1N0 rate
- Event-free survival
- Overall survival
- Quality of life outcomes
- Safety
The baseline characteristics of the SURE-01 patient cohort are summarized below. Thirty-eight patients were screened between March 2022 and May 2024, and 31 of these patients received neoadjuvant SG. Three patients died during the neoadjuvant treatment, 18 completed treatment with pathologic response assessment, and 10 patients are receiving ongoing neoadjuvant therapy. The median patient age was 70 years. Of the 21 patients, 76% were cisplatin refusing and 24% were cisplatin ineligible. Two-thirds of the cohort had cT2N0 disease. Almost half of the patients (48%) had urothelial carcinoma mixed with other variant histologies.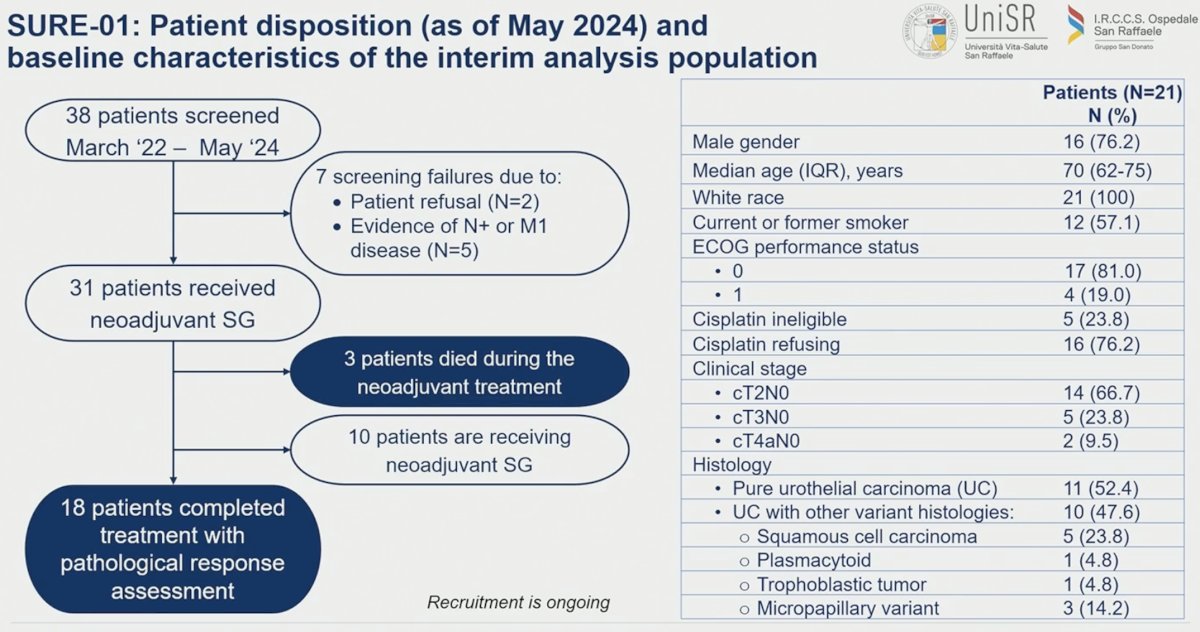
The study protocol was amended after the initial eight patients experienced treatment-related adverse events:
- Grade 3–4 neutropenia: 6/8 (75%)
- Timing of grade 3-4 events onset: after C1D8 in 5/6 patients
- Grade 3-4 diarrhea: 4/8 (50%; all after C1D8).
- Dose reductions of SG: 6/8 (75%), the remaining two patients had treatment discontinuation/death
- One treatment-related grade 5 event (sepsis).
- One treatment-unrelated Grade 5 event.
The dose of SG was reduced to 7.5 mg/kg from cycle 1 day 1. G-CSF use was introduced as a primary prophylactic measure from cycle 1 day 9. Patients who had ≥3 risk factors for febrile neutropenia (per ASCO guidelines) were excluded from trial enrolment.
In the overall cohort of 21 patients, any grade treatment-related adverse event was observed in 81% of patients. Grade 3 and 4 treatment-related adverse events were noted in 33.3% and 19.1% of patients. One patient experienced a treatment-related death (grade 5 event). The most common grade ≥3 treatment-related adverse events were neutropenia (33%) and diarrhea (24%).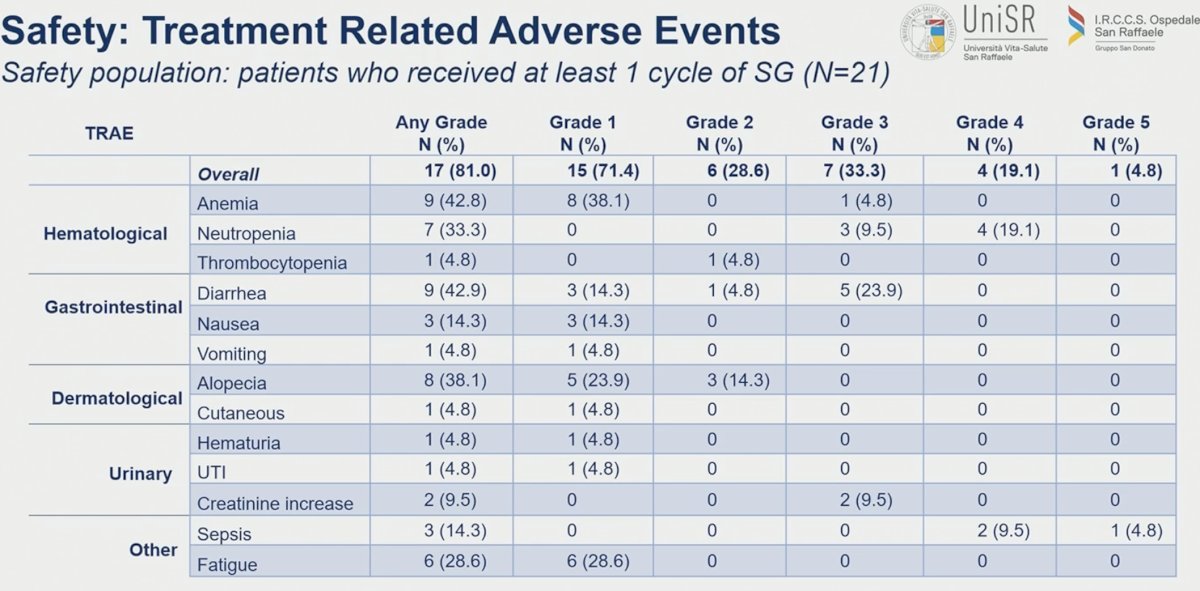
Three patients died after treatment discontinuation at C1D8:
- One death was due to sepsis following Grade 4 neutropenia + Grade 3 diarrhea.
- Two deaths due to non-treatment-related complications (CNS deterioration for unknown reason and disease progression, respectively)
- Grade 3-4 treatment-related adverse events were more common in patients with homozygous or heterozygous UGT1A1*28 polymorphisms (5/8; 62.5%) versus wild-type status (2/10; 20%).
Eighteen of 21 patients completed all four cycles of neoadjuvant SG and underwent surgery. Of these 18 patients, 11 underwent a radical cystectomy and 7 refused surgery due to evidence of a clinical complete response (n=6) or due to patient decision (subsequently underwent a repeat TURBT; n=1). The median duration of neoadjuvant treatment was 11.7 weeks, and the median time from end of SG to surgery was 6.9 weeks. A pathologic complete response was in 4/11 (36.4%) patients who underwent a radical cystectomy. An ypT≤1N0 response was observed in patients (45%). One patient had disease relapse/progression during or post-SG.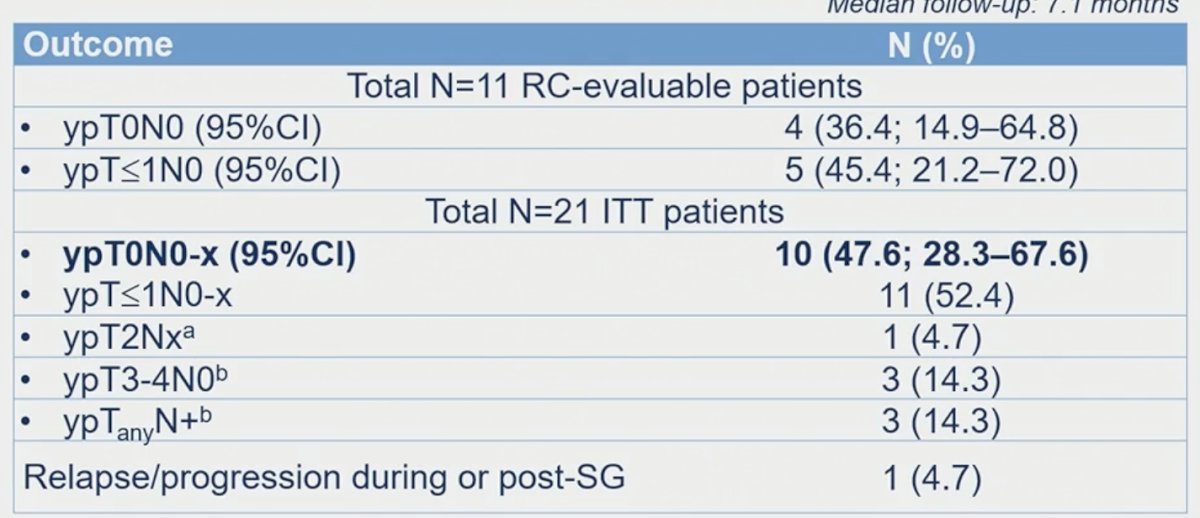
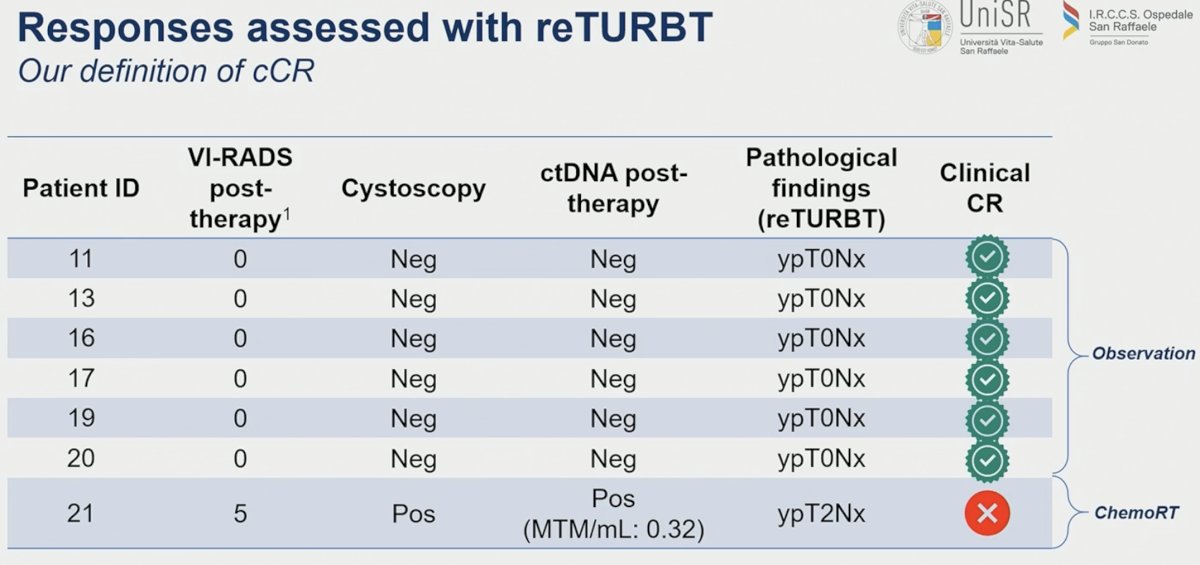
Correlative biomarker analyses demonstrated that all patients with residual ypT3-4N0 or ypTanyN+ disease (N=6) had a ctDNA-negative Signatera test post-radical cystectomy (none of them have relapsed). The remaining cDNA analyses of pre-post therapy associations are ongoing and immature.
Dr. Cigliola concluded as follows:
- The interim findings from the SURE-01 trial provide several useful information that may be considered for the development of preoperative SG in patients with MIBC:
- The interim ypT0N0 rate and safety profile post-protocol amendment give reassurance for the continuation of the study.
- A few patients meeting rigorous criteria of clinical complete response, who elected to undergo a bladder-saving approach, revealed a potential for cure (ypT0Nx stage, ctDNA-)
- Study amendments that included changing the dose from 10 mg/kg to 7.5 mg/kg, requiring primary prophylaxis with PegG-CSF starting at C1D9, and excluding patients at high risk for neutropenia led to an improved safety profile.
Presented by: Antonio Cigliola, MD, Medical Oncology Department, IRCCS San Raffaele Hospital, Milan, Italy
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:- Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol. 2021;39(22): 2474-85.
- Loriot Y, Petrylak DP, Kalebasty AR, et al. TROPHY-U-01, a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors: updated safety and efficacy outcomes. Ann Oncol. 2024;35(4): 392-401.


