(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a kidney and bladder rapid oral abstract session. Dr. Thomas Powles presented the results of an ad hoc quantitative analysis of circulating tumor DNA (ctDNA) from the phase 3 KEYNOTE-361 trial of pembrolizumab versus platinum-based chemotherapy for patients with advanced urothelial carcinoma.
KEYNOTE-3611 was a randomized, open-label, phase 3 trial of adult patients with untreated, locally advanced, unresectable, or metastatic urothelial carcinoma who were randomized to:
- Pembrolizumab + chemotherapy (gemcitabine + carboplatin or cisplatin)
- Pembrolizumab monotherapy
- Chemotherapy alone
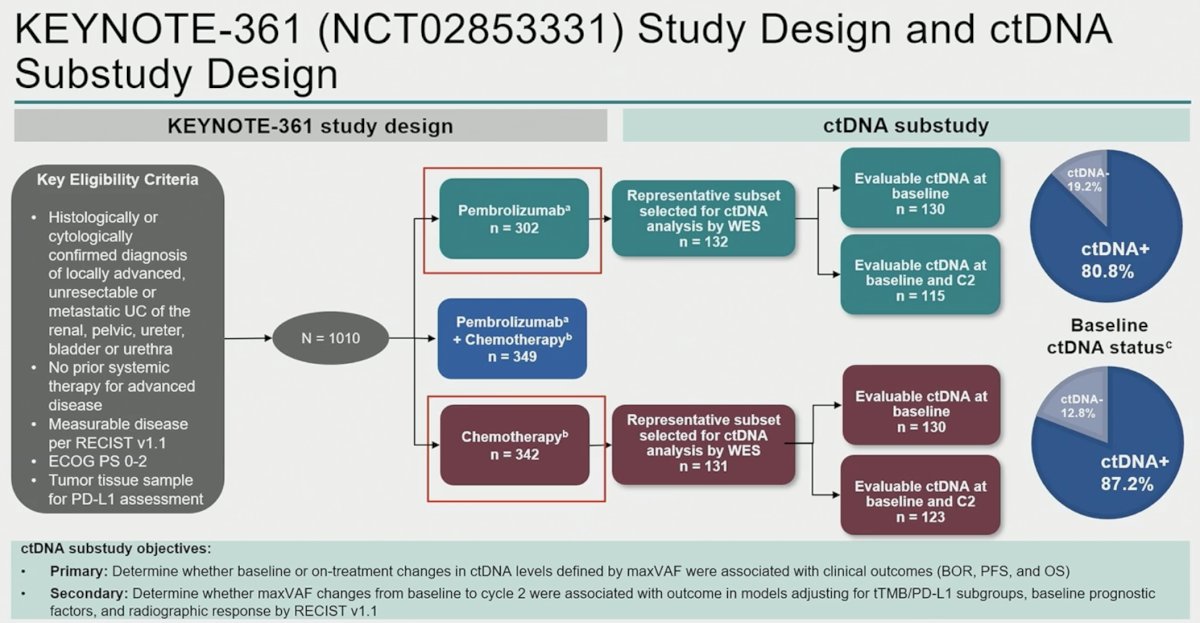
In this ad hoc analysis, Dr. Powles and colleagues performed ctDNA subanalyses on patients from the monotherapy arms (pembrolizumab only; chemotherapy only). The primary study objectives were as follows:
- Primary: Determine whether baseline or on-treatment changes in ctDNA levels defined by maximum detected variant allele frequency (maxVAF) were associated with clinical outcomes (BOR. PFS, and OS)
- Secondary: Determine whether maxVAF changes from baseline to cycle 2 were associated with outcomes in models adjusting for tTMB/PD-L1 subgroups, baseline prognostic factors, and radiographic response by RECIST v1.1.
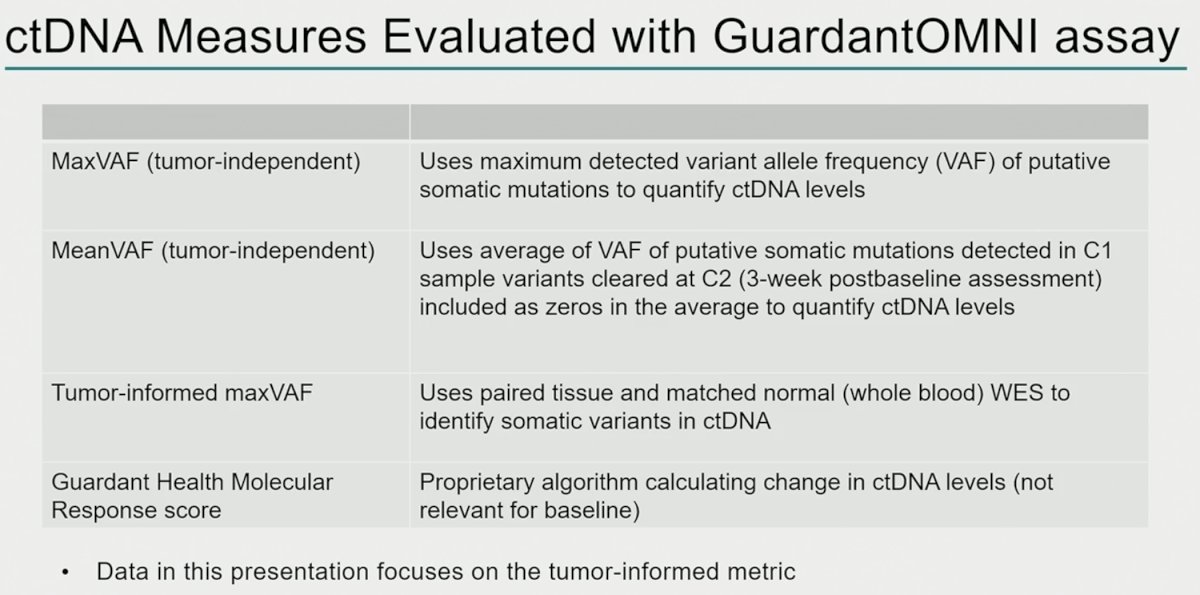
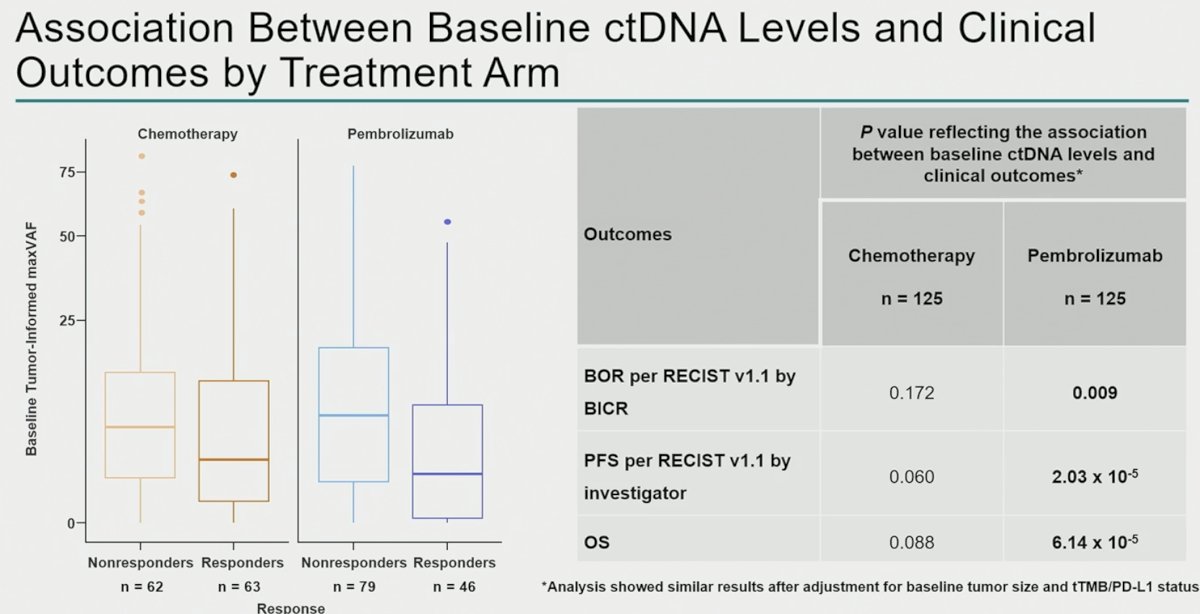
ctDNA samples from 263 patients (chemotherapy, n=131; pembrolizumab, n=132) were analyzed. Clinical characteristics and baseline ctDNA levels within arms were similar. Lower C1 maxVAF was associated with improved objective response rate, progression-free survival, and overall survival in the pembrolizumab arm (p< 0.01) and was robust to adjustment for TMB and PD-L1, but not in the chemotherapy arm (p> 0.05).
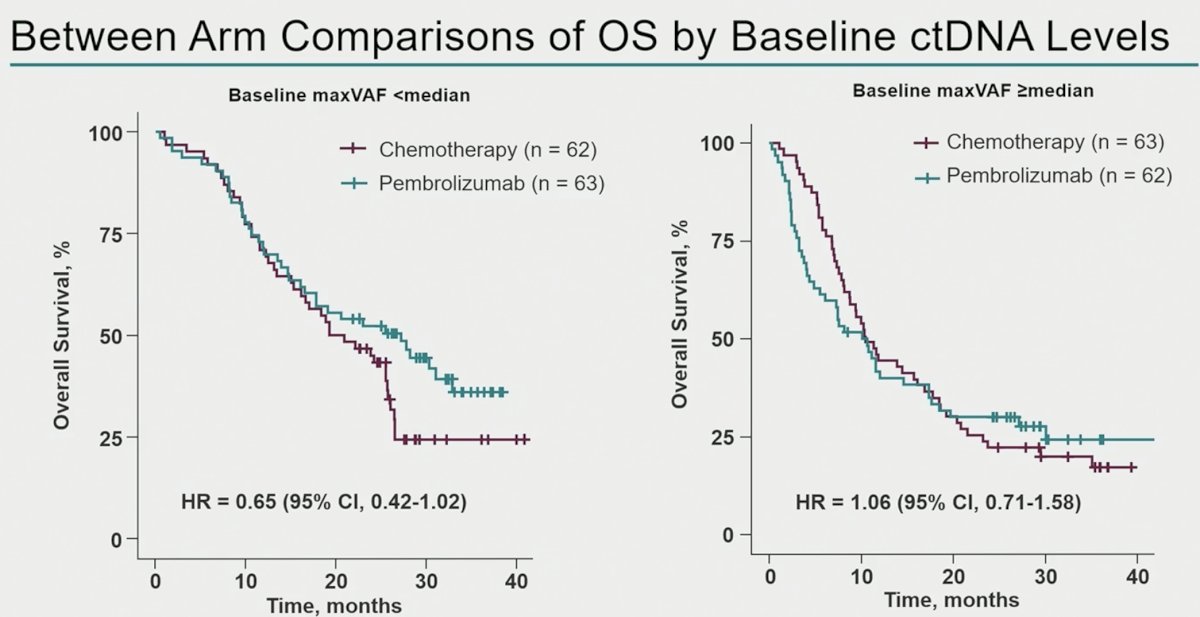
Between arm comparisons of overall survival outcomes by baseline ctDNA levels demonstrated that in patients with baseline maxVAF <median, patients treated with pembrolizumab had superior overall survival outcomes, compared to those treated with chemotherapy alone (HR: 0.65, 95% CI: 0.42–1.02). Conversely, among patients with baseline maxVAF ≥median, there were no between arm differences in overall survival rates.
A larger proportion of patients in the chemotherapy arm achieved ctDNA clearance from cycle 1 to cycle 2 (41% versus 11%):
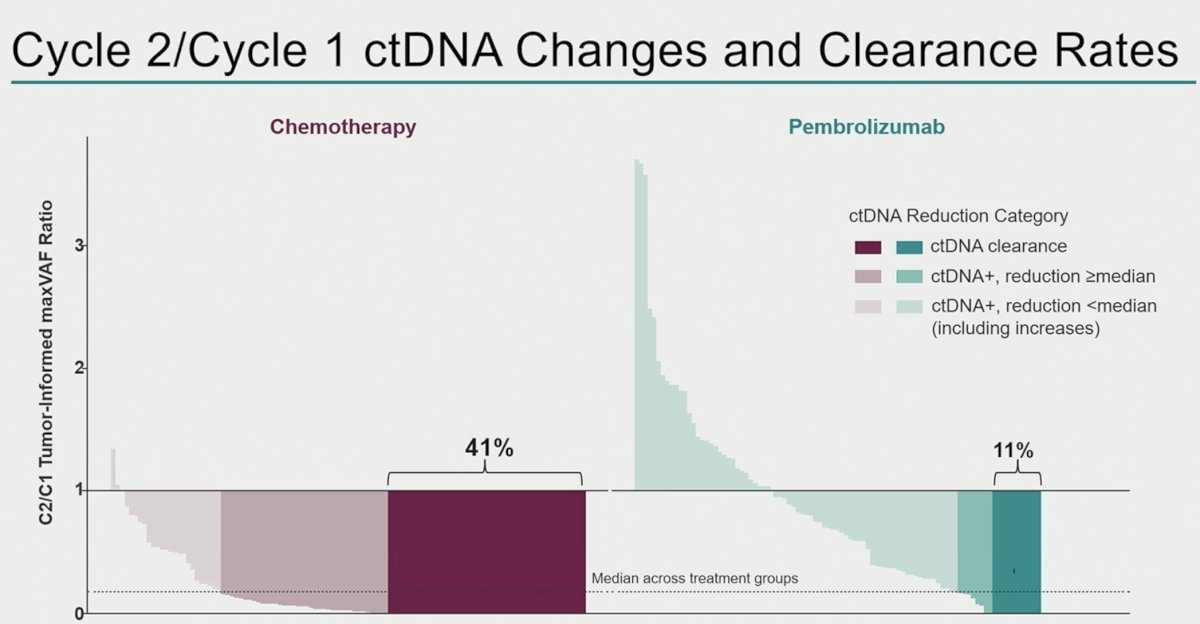
Next, the investigators correlated ctDNA changes with radiographic changes. In the chemotherapy arm, ctDNA reductions were observed across the spectrum of patients experiencing radiologic tumor volume changes. Conversely, in the pembrolizumab arm, ctDNA clearances were concentrated in those patients with deep radiologic response.

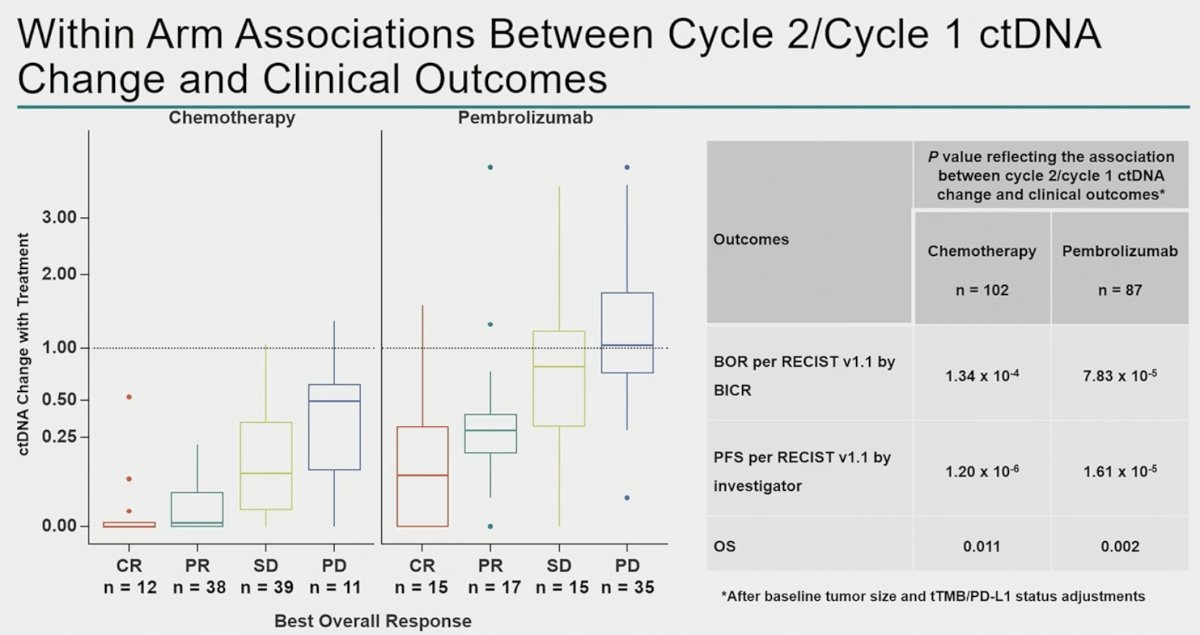
This is further reflected in the box and whisker plot below, which demonstrates that ctDNA clearance is observed almost independently of radiographic response in the chemotherapy arm, whereas ctDNA clearance is almost exclusively seen in pembrolizumab-treated patients with radiographic complete or partial responses.
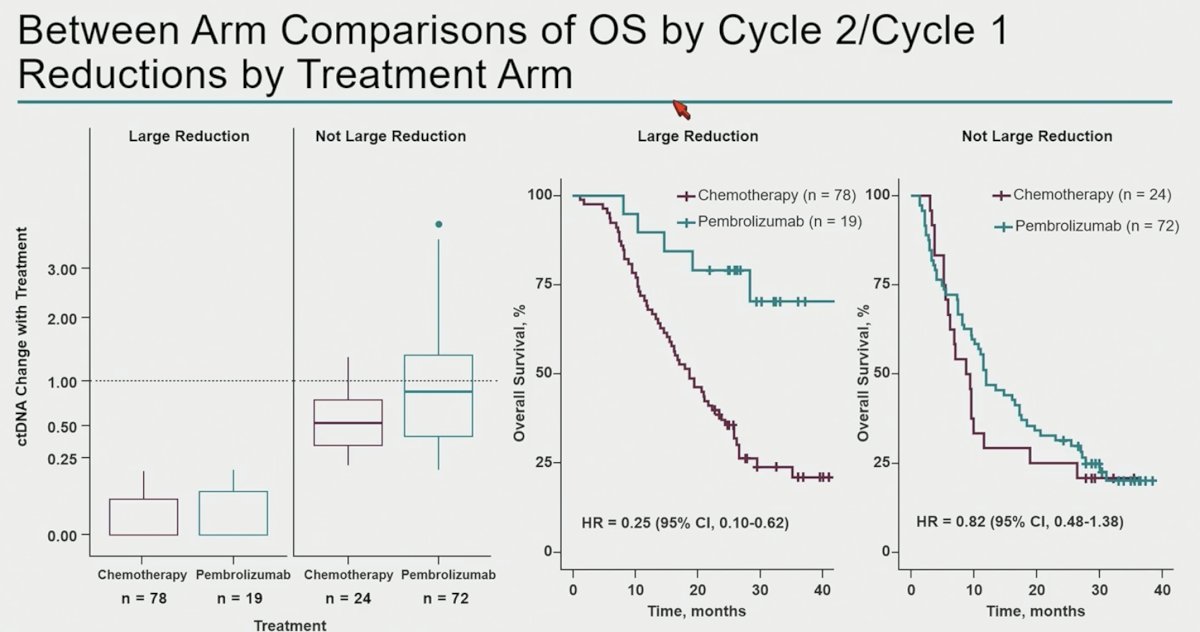
These findings are further emphasized by the Kaplan Meier curves below which demonstrate that overall survival outcomes are superior for pembrolizumab-treated patients with large reductions in ctDNA levels (i.e., ctDNA reductions were more ‘meaningful’ in pembrolizumab arm; HR: 0.25, 95% CI: 0.10–0.62).
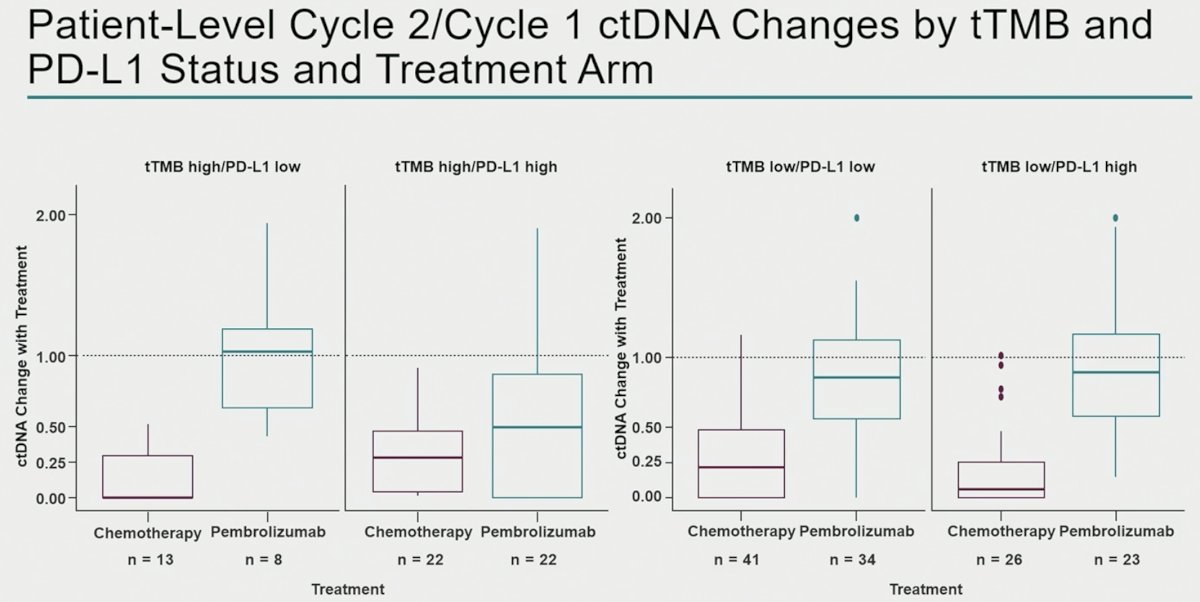
The most significant decreases in ctDNA levels among pembrolizumab-treated patients were observed in the TMB high/PD-L1 high patients, whereas ctDNA changes were observed in chemotherapy-treated patients irrespective of TMB/PD-L1 status.
Dr. Powles concluded as follows:
- In this retrospective, exploratory analysis of KEYNOTE-361, most patients were positive for ctDNA at baseline.
- Baseline ctDNA levels were associated with clinical outcomes for pembrolizumab but not for chemotherapy.
- Chemotherapy induced larger ctDNA decreases from baseline to treatment cycle 2 than pembrolizumab (including cDNA clearance)
- Changes with pembrolizumab were more strongly associated with long-term outcomes and were enriched with higher tTMB and PD-L1 levels.
- Short-term ctDNA reductions were not treatment-independent surrogates for clinical outcomes.
- Patients with larger ctDNA reductions in the pembrolizumab arm had a much more favorable overall survival relative to patients with larger ctDNA reductions in the chemotherapy arm.
- Data presented herein have demonstrated that ctDNA dynamics can be influenced by the mechanisms of treatment, which is relevant for consideration of this biomarker in future combination therapies.
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Director, Barts Cancer Centre at St. Bartholomew's Hospital, London, UK
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Powles T, Csoszi T, Ozguroglu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomized, open-label, phase 3 trial. Lancet Oncol. 2021 May 26;S1470-2045(21)00152-2.


