(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a kidney and bladder cancers trials-in-progress poster session. Dr. Bassel Nazha presented an ongoing phase II study evaluating the combination of enfortumab vedotin plus pembrolizumab for the treatment of locally advanced or metastatic bladder cancer of variant histology.
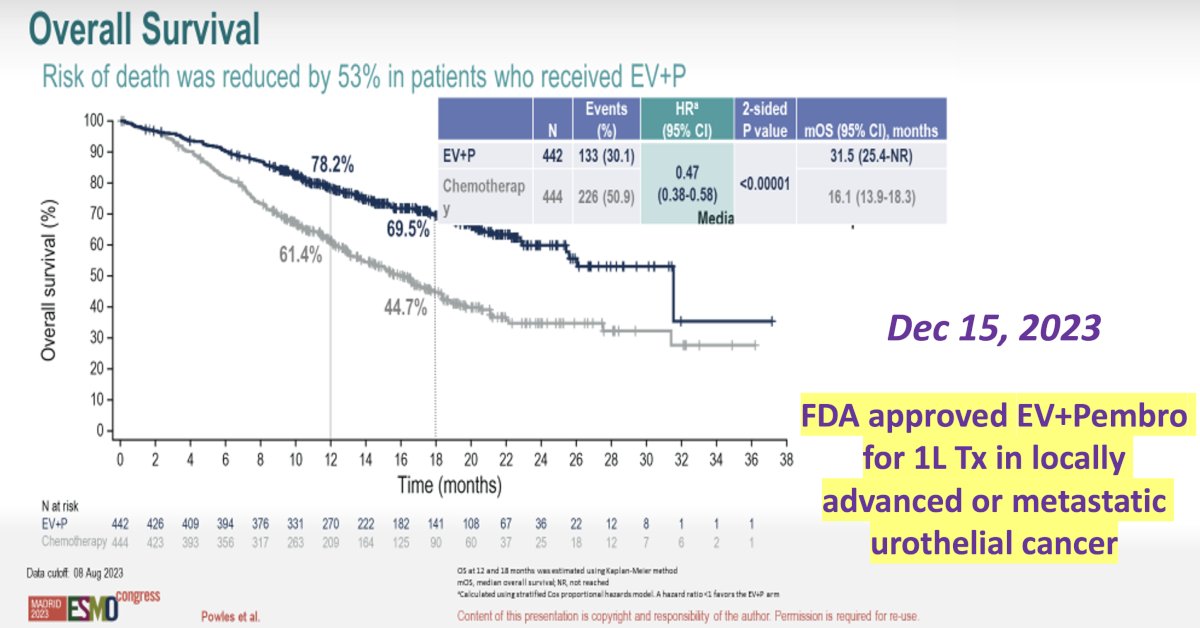
In EV-302/KEYNOTE-A39, the combination of enfortumab vedotin, a Nectin-4 directed antibody-drug conjugate, and pembrolizumab, a PD-1 inhibitor, was associated with a two-fold improvement in median overall survival, compared to platinum-based combination chemotherapy (32 versus 16 months).1 Notably, this trial allowed for the inclusion of patients with either pure urothelial carcinoma (85%), mixed type (11.6%), or pure variant disease (1.3%).
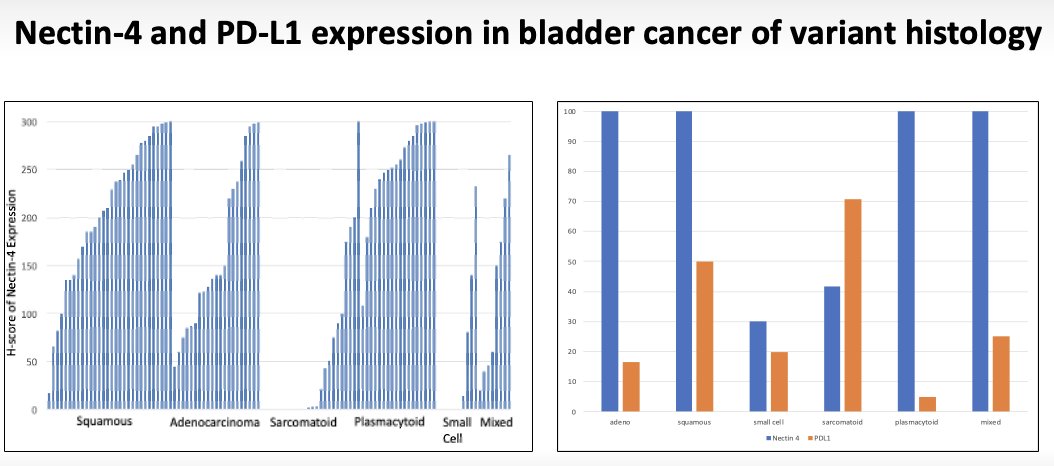
Bladder cancer of variant histology classically accounts for up to 25% of bladder tumors. Patients with variant bladder histology or non-urothelial bladder cancers are largely under-represented or excluded from phase III trials for urothelial cancer (only 1.3% of EV-302/KEYNOTE-A39 cohort). Significantly, Nectin-4 and PD-L1 are both expressed in non-urothelial and variant histology tumors.2 This suggests that these patients may also derive a benefit from enfortumab vedotin pembrolizumab combination therapy.

*Slide adapted from Dr. Eila Skinner’s presentation at ASCO GU 2022.
This is a phase II, open-label, single-center trial at the Winship Cancer Institute of Emory University (NCT05756569). The trial is currently open for enrollment. The study design is illustrated below. This trial is recruiting patients with advanced or metastatic disease with either: (i) non-urothelial bladder cancer, (ii) variant histology bladder cancer, (iii) or mixed histology if predominant histology (≥50%) is variant or non-urothelial. In the 1st stage, 15 patients will receive pembrolizumab 200 mg IV every three weeks plus enfortumab vedotin 1.25 mg/kg on days 1 and 8 of each 21-day cycle. If a response is observed in ≥2/15 patients, then the 2nd stage will enroll an additional 10 participants (total n=25). The maximum duration of enfortumab vedotin + pembrolizumab is two years. The enfortumab vedotin dose is capped at 125mg.
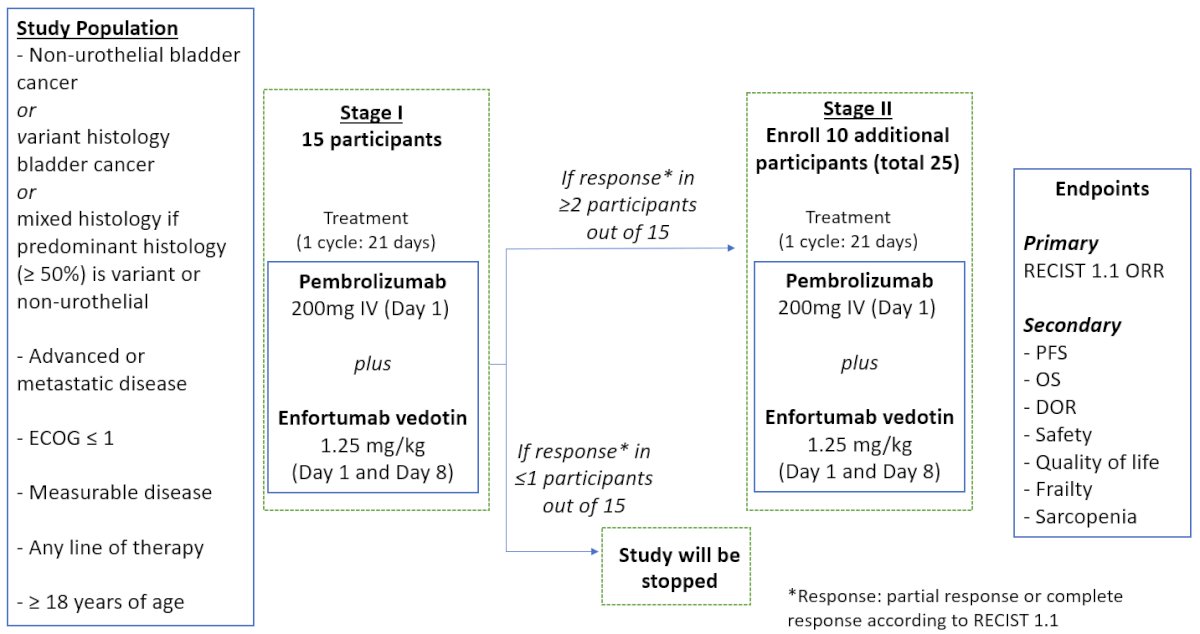
The primary study endpoint is objective response rate (partial or complete), per RECIST v1.1. Secondary outcomes include:
- Progression-free survival
- Overall survival
- Duration of response
- Safety
- Quality of life
- Frailty
- Sarcopenia
- Variant histology bladder cancer: nested, microcytic, micropapillary, lymphoepithelioma-like, plasmacytoid, giant cell, poorly differentiated, lipid-rich, clear cell, sarcomatoid.
- Non-urothelial bladder cancer of epithelial origin including squamous cell carcinoma and adenocarcinoma (urachal and non-urachal).
- Tumors of the ureter, urethra, urachus, or renal pelvis are included.
Notable exclusion criteria are as follows:
- Neuroendocrine bladder cancer
- Non-epithelial cancers (e.g. sarcoma, lymphoma)
- Prior immune checkpoint inhibitor and/or enfortumab vedotin treatment
- Active autoimmune disease
- Uncontrolled diabetes mellitus
- CHF NYHA Class 3 or 4, unstable angina, serious cardiac arrythmias within 6 months.
Presented by: Bassel Nazha, MD, MPH, Assistant Professor, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024;390(10): 875-88.
- Case K, Martini DJ, Dababneh M, et al. Expression of Nectin-4 and PD-L1 in bladder cancer with variant histology. J Clin Oncol. 2022;40(Suppl 6).


