(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL was host to the Poster Session: Genitourinary Cancer: Kidney and Bladder. Dr. Peter H. O'Donnell presented the study EV-103: Neoadjuvant treatment with enfortumab vedotin monotherapy in cisplatin-ineligible patients with muscle invasive bladder cancer (MIBC)—2-year event-free survival and safety data for Cohort H.
There have been few advances addressing the high unmet need for neoadjuvant treatment options in patients with MIBC who are cisplatin-ineligible and undergoing radical cystectomy and pelvic lymph node dissection (RC+PLND). To date, no neoadjuvant treatment options have been shown to improve survival in this population.
Enfortumab vedotin (EV) is an antibody-drug conjugate directed to Nectin-4, which is highly expressed in urothelial cancer cells, the proposed mechanism of action is shown in the figure below). EV, alone and in combination with pembrolizumab, has been shown to improve OS versus chemotherapy in patients with previously treated and untreated locally advanced or metastatic urothelial cancer.1,2
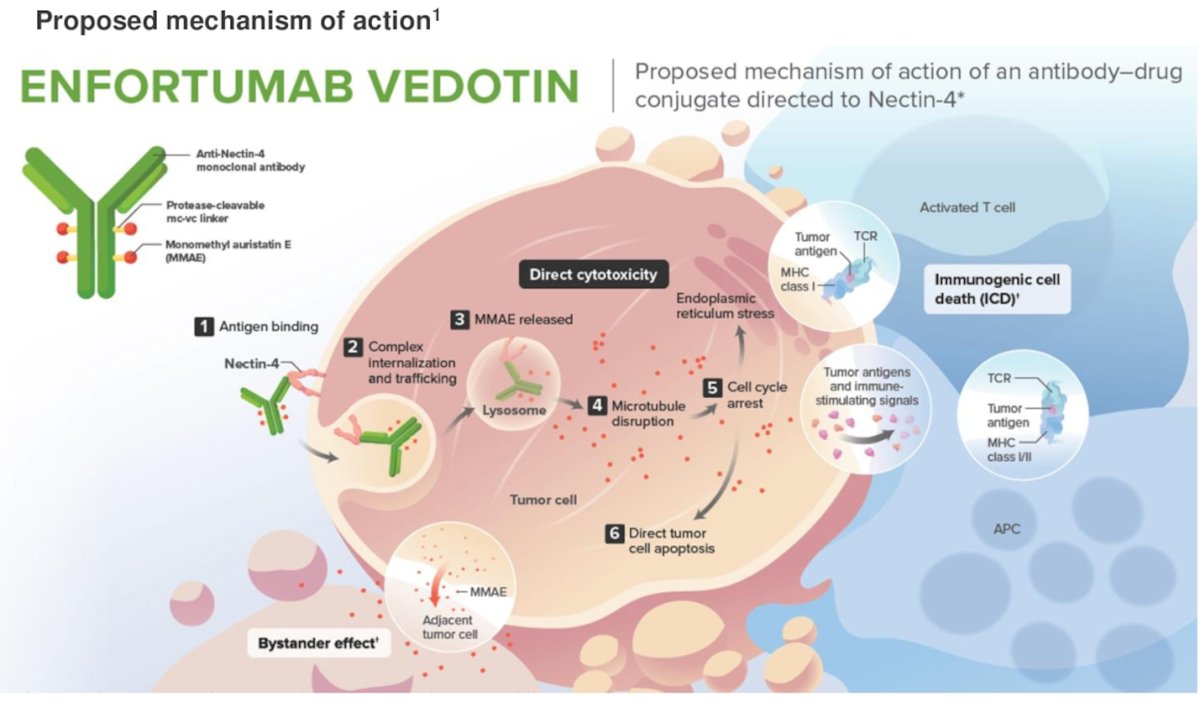
Cohort H of the EV-103 phase 1b/2 study (NCT03288545) enrolled cisplatin-ineligible patients with MIBC (T2-T4aN0M0) and ECOG PS ≤2 who were eligible for radical cystectomy and pelvic lymph node dissection. Patients received neoadjuvant enfortumab vedotin (1.25 mg/kg) on Days 1 and 8 every 21 days for 3 cycles before undergoing radical cystectomy and pelvic lymph node dissection. The trial design for EV-103 cohort H is illustrated below:
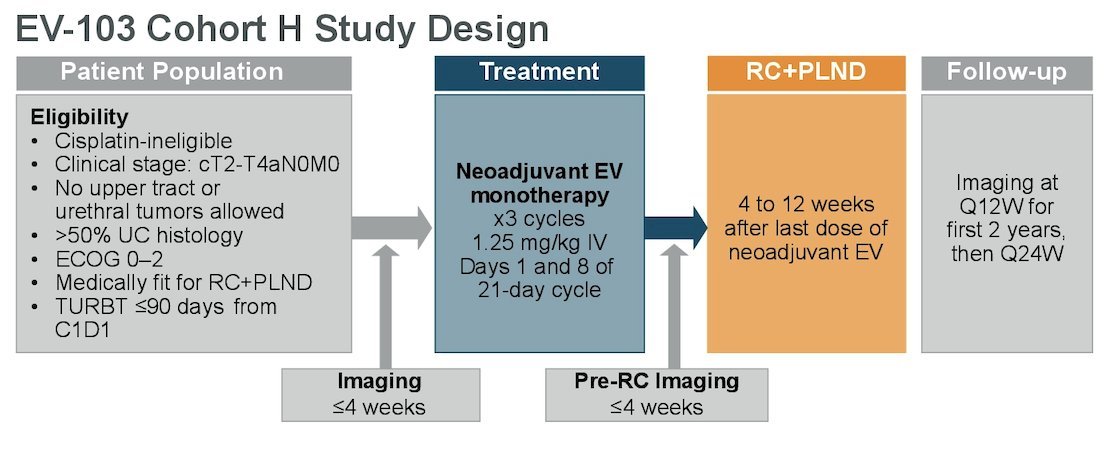
The primary endpoint was pathological complete response (pCR) rate (ypT0 and N0) by central pathology review. Key secondary endpoints included pathological downstaging (pDS) rate (ypT0, ypTis, ypTa, ypT1, and N0), safety, and event-free survival (EFS) per investigator assessment (radiographic progression prior to RC, failure to undergo RC, gross residual disease at time of RC, recurrence, or death).
During ASCO 2023, Dr. Flaig presented promising results, including a 1-year event-free survival (EFS) of 76.4% (95% CI, 52.2, 89.4), a pathological complete response (pCR) rate of 36.4% (95% CI, 17.2, 59.3), and a pathological downstaging rate of 50.0% (95% CI, 28.2, 71.8). In this presentation, Dr. O'Donnell reported updated results, including 2-year EFS.
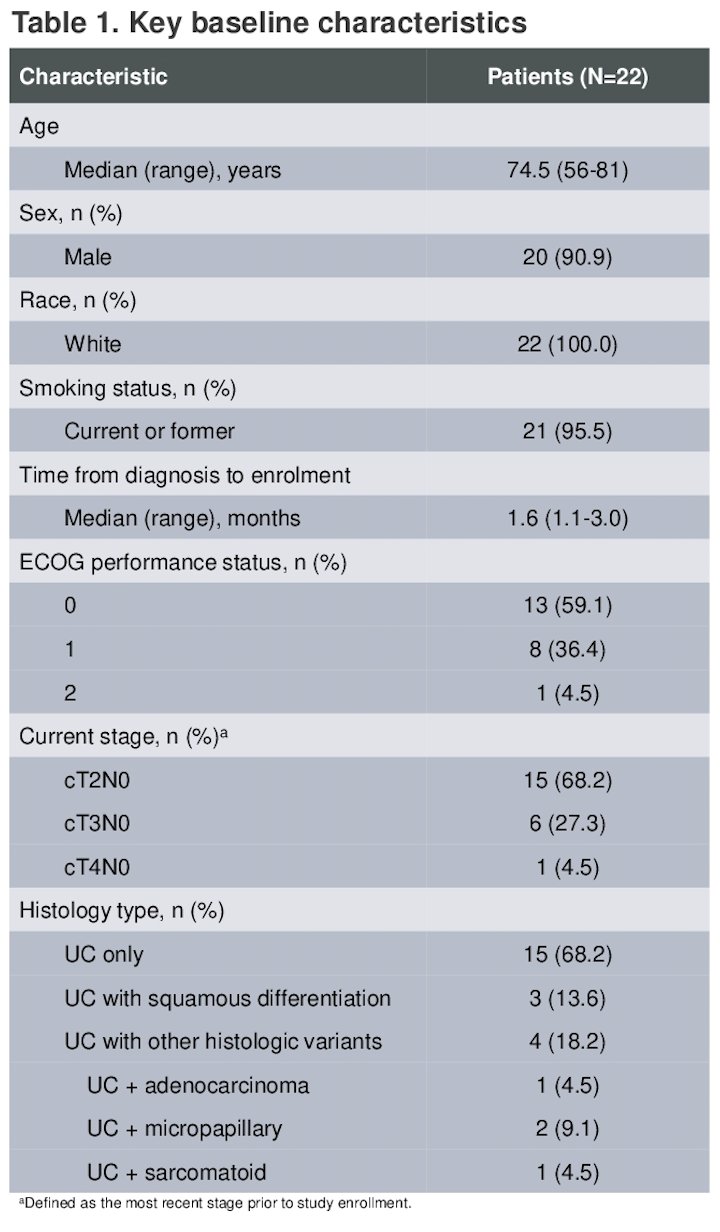
There were 22 patients enrolled and treated in the EV-103 study. The median age was 74.5 years (range 56-81). Fifteen patients had cT2 (68.2%), cT3 (27.3%), or cT4 (4.5%) disease. Sixty-eight percent of patients had urothelial carcinoma only; 31.8% had a mixed histology. Other baseline characterisctis are shown in the table below.
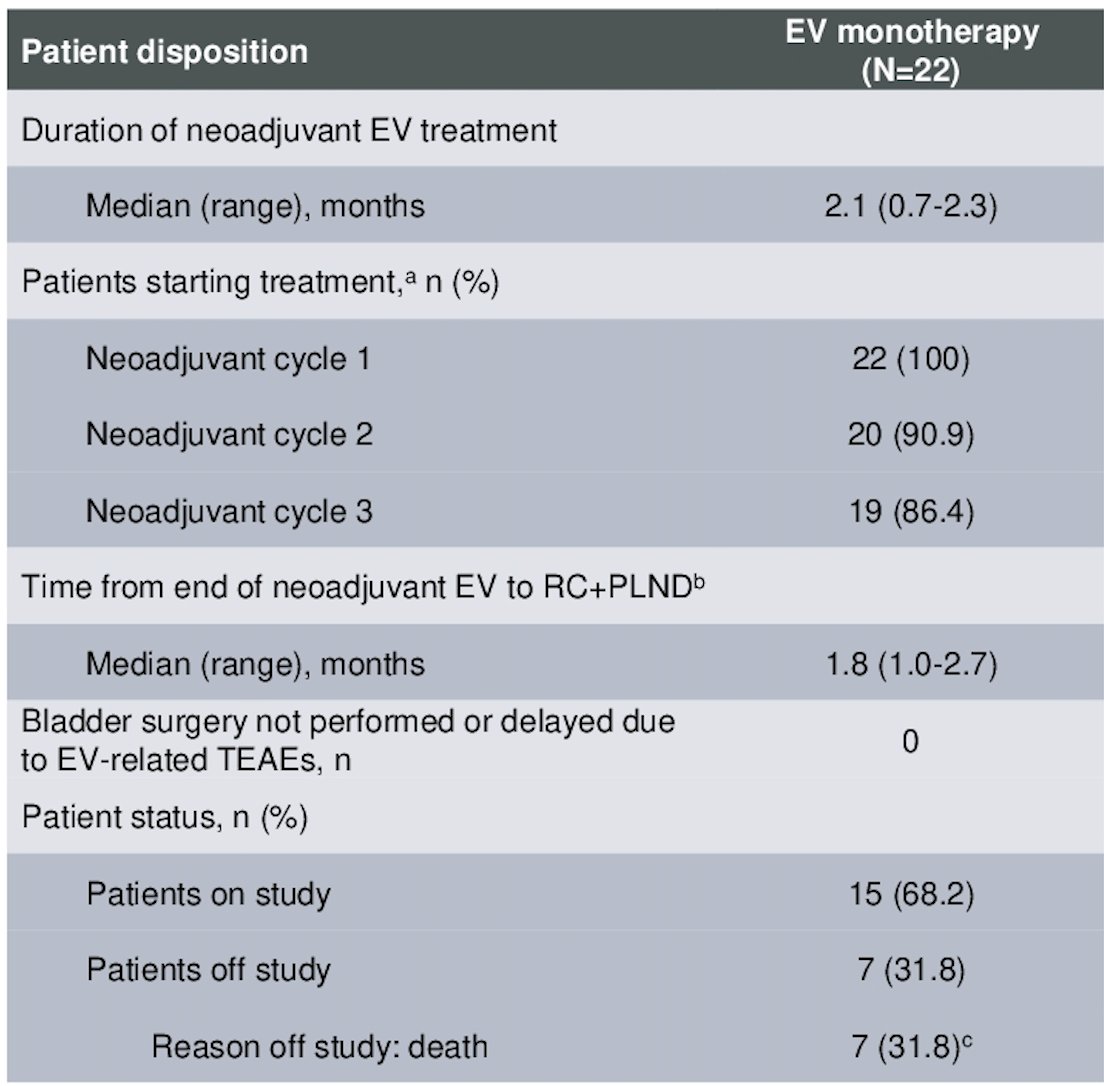
There were 86.4% of patients who completed all 3 cycles of EV treatment, with a median duration of enfortumab vedotin treatment of 2.1 months (range 0.7-2.3). All patients underwent surgery with no delays due to enfortumab vedotin-related treatment-emergent adverse events, the median time from end of neoadjuvant therapy to RC+PLND was 1.8 months. The patient disposition characteristics are depicted in the table below.
The pCR rate was 36.4% (95% CI, 17.2-59.3) and the pDS rate was 50.0% (95% CI, 28.2-71.8).
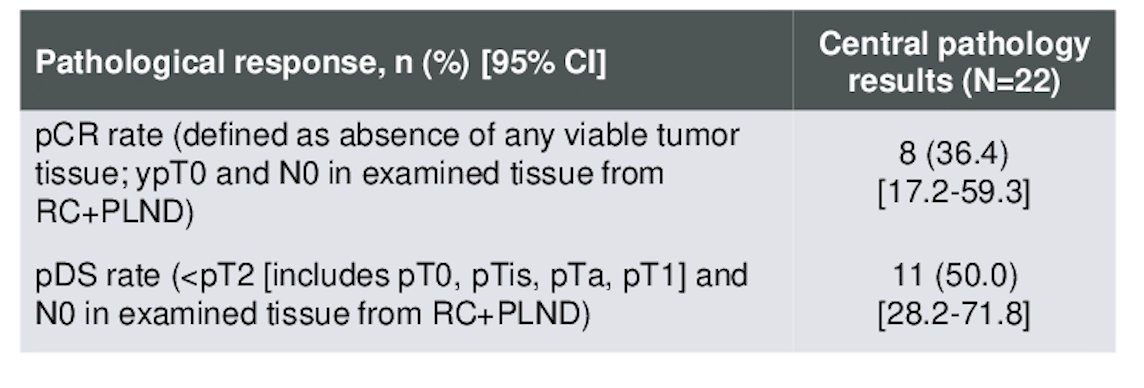
Dr. O'Donnell discussed that the most important data for this trial was EFS. The EFS rate at 24 months was 62.0% (95% CI, 38.2-78.9). Median EFS has not been reached to date for the overall population or for the patients who achieved a pCR. However, the median EFS for patients who did not achieve a pCR was 18.8 months. Kaplan-Meier graphics are shown below.
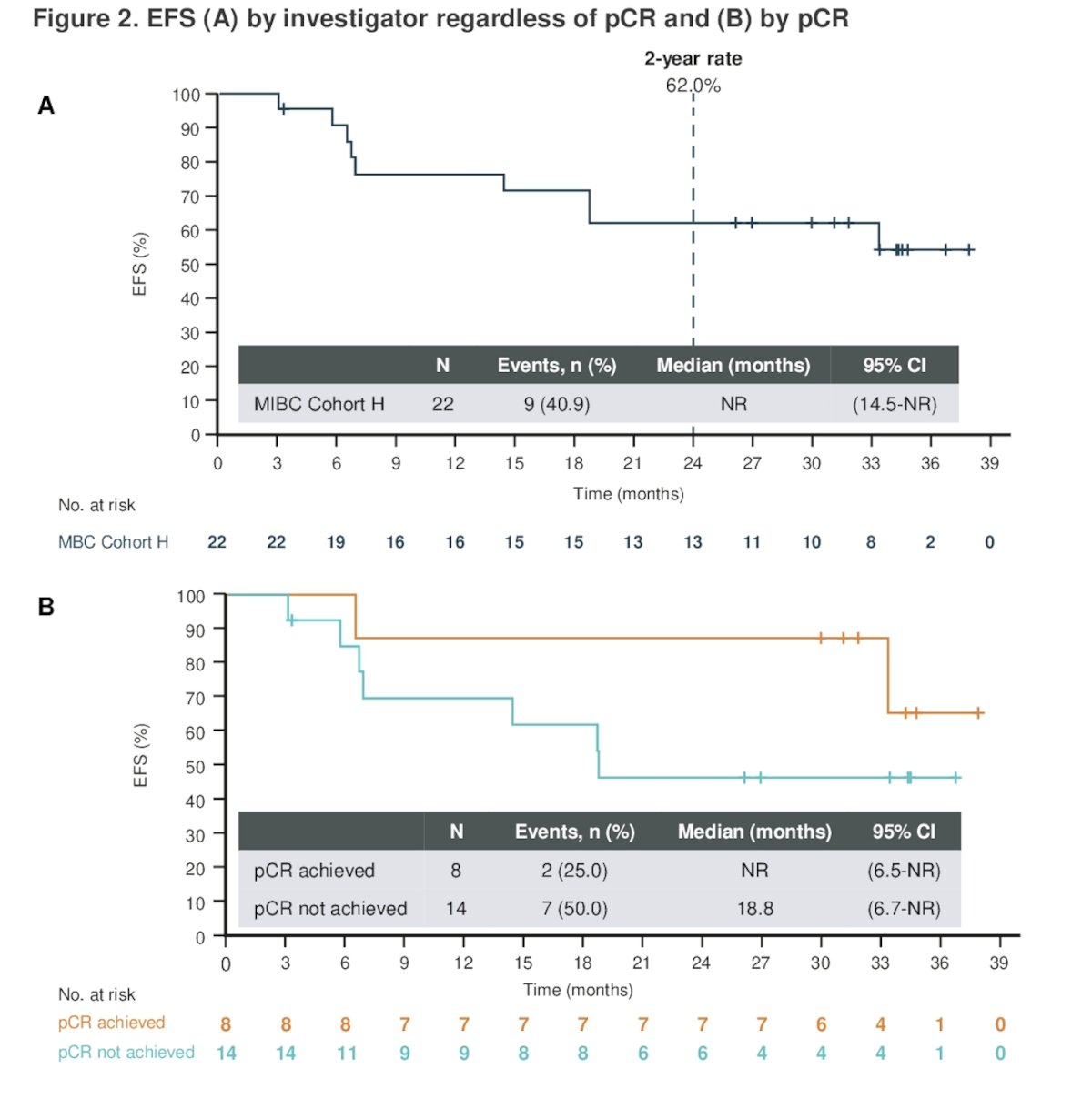
Moreover, 36.4% of patients received subsequent cancer-related therapy, the most common adjuvant therapy for residual MIBC after RC was Pembrolizumab (9.1%) as summarized below:
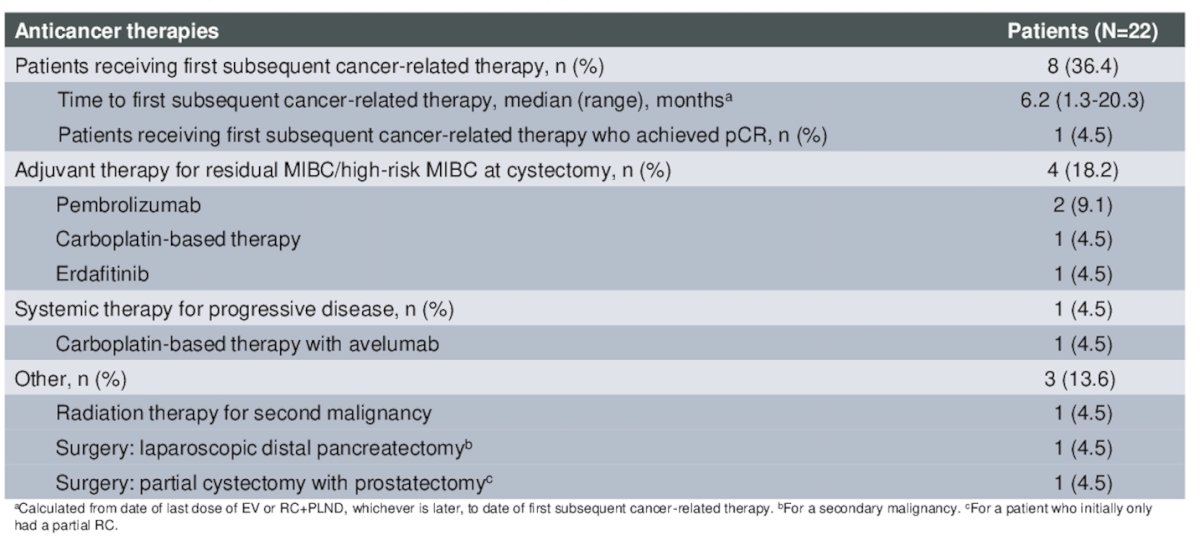
The most common surgery-related treatment emergent adverse events (TEAEs) were procedural pain (18.2%), anemia (13.6%), and constipation (13.6%) as illustrated in the graphic below.
The most common enfortumab vedotin-related TEAEs were fatigue (45.5%), dysgeusia (36.4%), and alopecia (31.8%). Overall, 18.2% of patients had grade ≥3 enfortumab vedotin-related treatment emergent adverse events, 68.2% of patients had surgery-related treatment emergent adverse events, and 31.8% patients had grade ≥3 surgery-related treatment emergent adverse events.
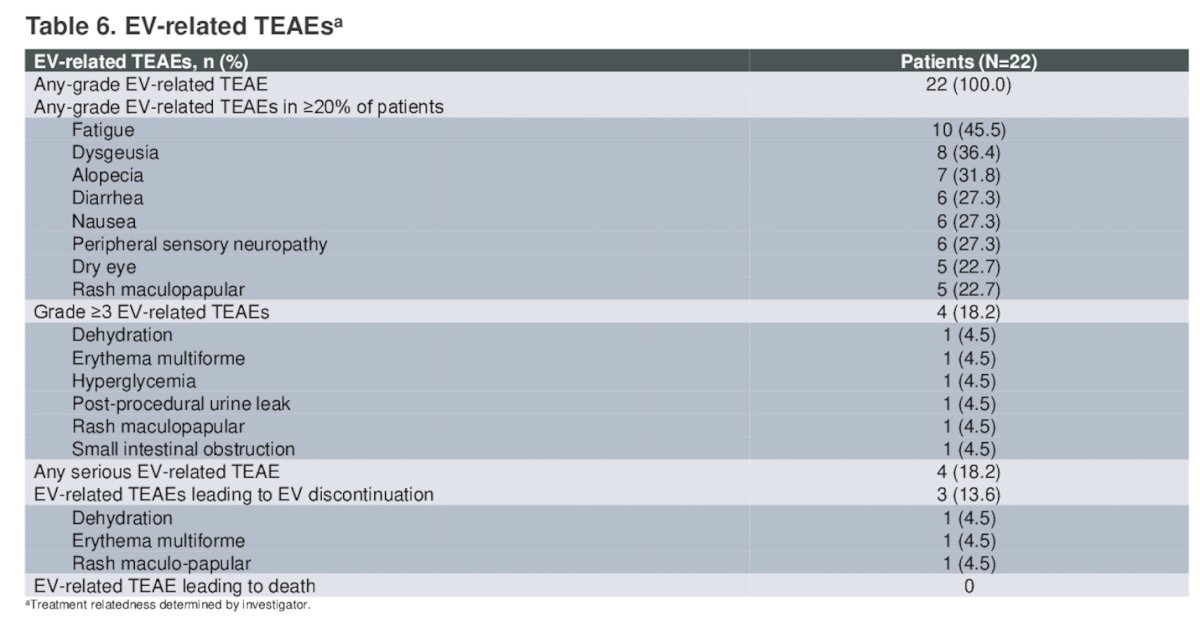
There were three patients who died due to adverse events (acute kidney injury, cardiac arrest, pulmonary embolism) unrelated to enfortumab vedotin treatment.
Dr. O'Donnell concluded his presentation with the following take-home messages:
- Neoadjuvant Enfortumab vedotin monotherapy demonstrated encouraging results for pathological response rate and event-free survival (62% at 2 years) with a manageable safety profile in cisplatin-ineligible patients with MIBC.
- All patients underwent surgery without delays due to neoadjuvant Enfortumab vedotin-related treatment-emergent adverse events (TEAEs) in this understudied population.
- These findings support ongoing phase 2 and 3 trials in MIBC evaluating Enfortumab vedotin alone or in combination with pembrolizumab in the neoadjuvant setting (EV-103 Cohort L, KEYNOTE-905/EV-303, KEYNOTE-B15/EV-304).
Presented by: Peter H. O'Donnell, MD, Medical Oncologist at the University of Chicago, Chicago, IL.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee JL, Matsubara N, Vulsteke C, Castellano D, Wu C, Campbell M, Matsangou M, Petrylak DP. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med. 2021 Mar 25;384(12):1125-1135. doi: 10.1056/NEJMoa2035807. Epub 2021 Feb 12. PMID: 33577729; PMCID: PMC8450892.
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.


