(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL was host to the Poster Session: Genitourinary Cancer: Kidney and Bladder. Dr. Michiel Simon Van Der Heijden presented a secondary analysis of the cisplatin (cis)-ineligible population from EV-302/KEYNOTE-A39 comparing Enfortumab vedotin (EV) with pembrolizumab (P) versus chemotherapy in previously untreated locally advanced or metastatic urothelial carcinoma (la/mUC).
Dr. Van Der Heijden began his presentation by emphasizing that for decades, the standard of care for first-line treatment of locally advanced/metastatic urothelial carcinoma (la/mUC) was cisplatin-based chemotherapy, with first-line treatment defined by cisplatin eligibility.1 Historically, patients not eligible for cisplatin have had a poorer prognosis compared to those who are eligible. Programmed death ligand 1 (PD-L1) inhibitors are available as maintenance therapy for a subset of patients or as second-line therapy for cisplatin-ineligible patients.2,3 More recently, the addition of nivolumab to cisplatin-gemcitabine has improved overall survival in the cisplatin-eligible population.4
There have been very few advances addressing the high unmet need in advanced urothelial cancer, specifically for the cisplatin-ineligible population, with the median overall survival hovering around 13 months over the last few decades.1 Recently, the combination of EV+P has been approved by the FDA for the treatment of locally advanced/metastatic urothelial carcinoma based on a favorable benefit/risk versus platinum-based chemotherapy in EV-302/KEYNOTE-A39 (NCT04223856), and a clinically meaningful benefit in comparison with chemotherapy for progression-free survival (PFS) (HR: 0.45, P<0.00001) and overall survival (OS) (HR: 0.47, P<0.00001).5
In this report, Dr. van der Heijden presented the results of the cisplatin-ineligible subgroup analyses from the EV-302 study.
In EV-302, patients with previously untreated la/mUC were randomized 1:1 to receive 3-week cycles of EV (1.25 mg/kg IV; Days 1 and 8) and pembrolizumab (200 mg IV; Day 1) or platinum-based chemotherapy (gemcitabine with cisplatin or carboplatin). Patients were deemed eligible/ineligible for cisplatin by the following criteria:
- Glomerular filtration rate (GFR) ≥30 to <60 mL/min (Cockcroft-Gault formula, MDRD, 24h urine)
- Hearing loss of grade 2 or higher (NCI CTCAE)
- Eastern Cooperative Oncology Group performance-status (ECOG-PS) score of 2
- New York Heart Association (NYHA) class III heart failure at enrollment
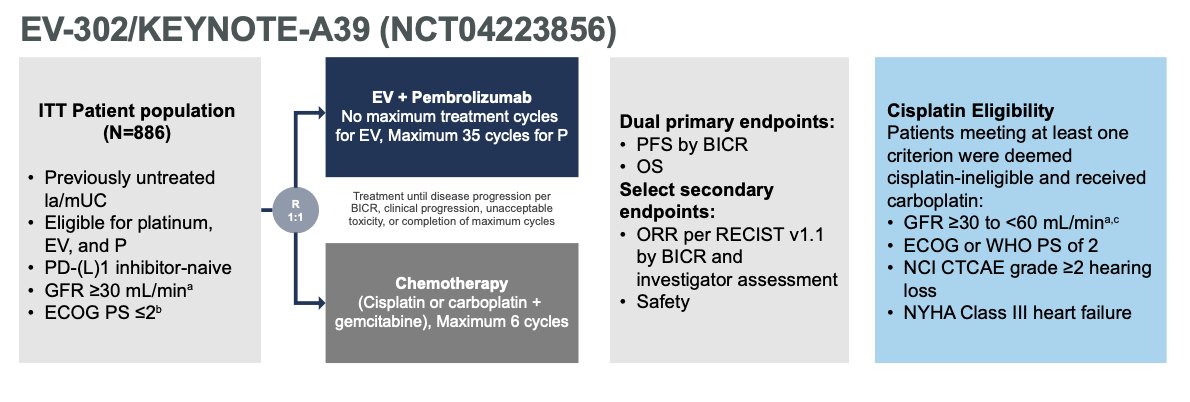
The study design is illustrated below. The dual primary endpoints were progression-free survival (PFS), assessed via blinded independent central review, and overall survival (OS)
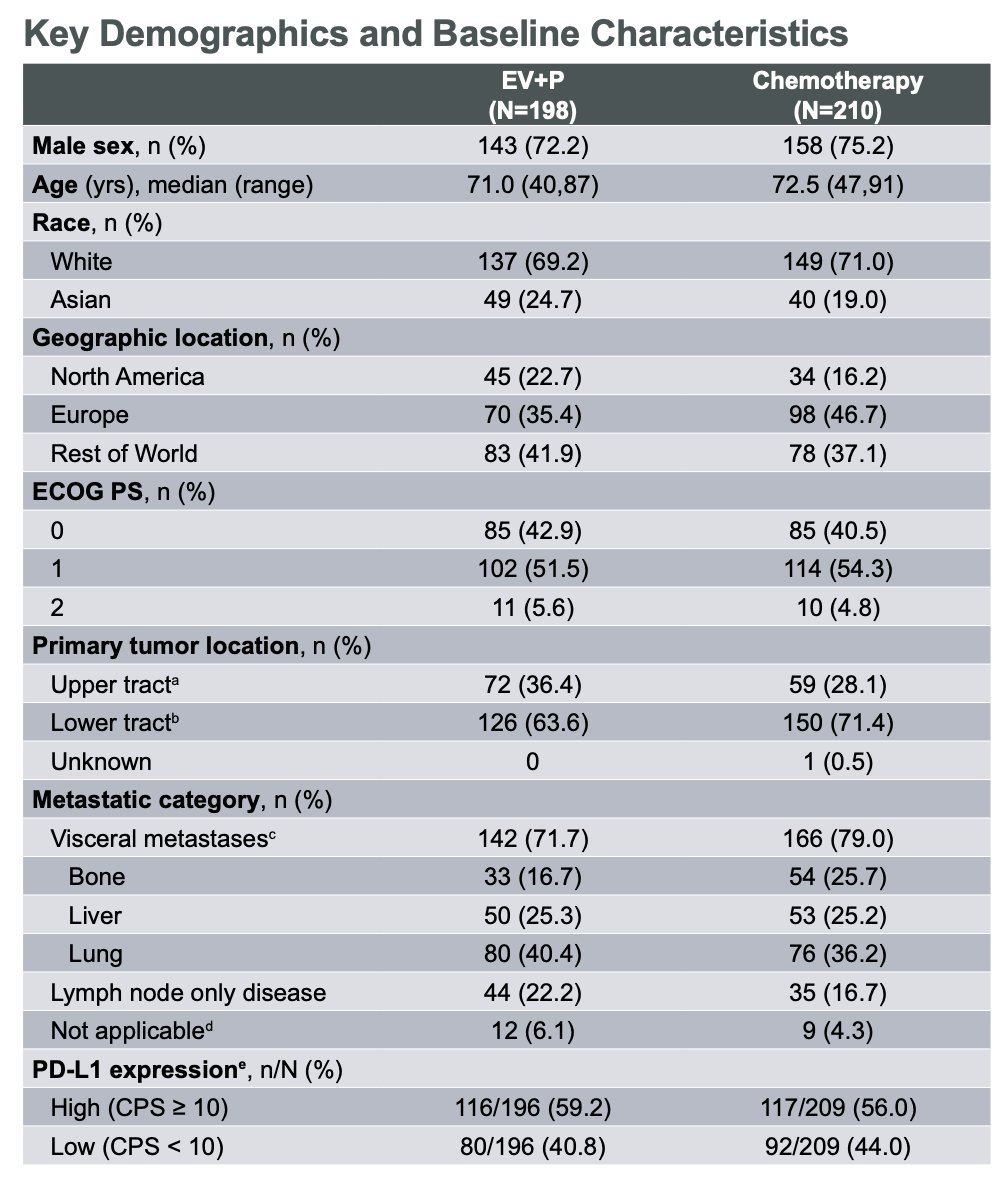
For this cisplatin-ineligible subgroup analysis of EV-302, 408 patients were included. Compared with the ITT population, cisplatin-ineligible patients were older, had higher ECOG PS scores, and had a higher rate of upper tract disease. The clinical demographic and baseline characteristics are illustrated in the table below.
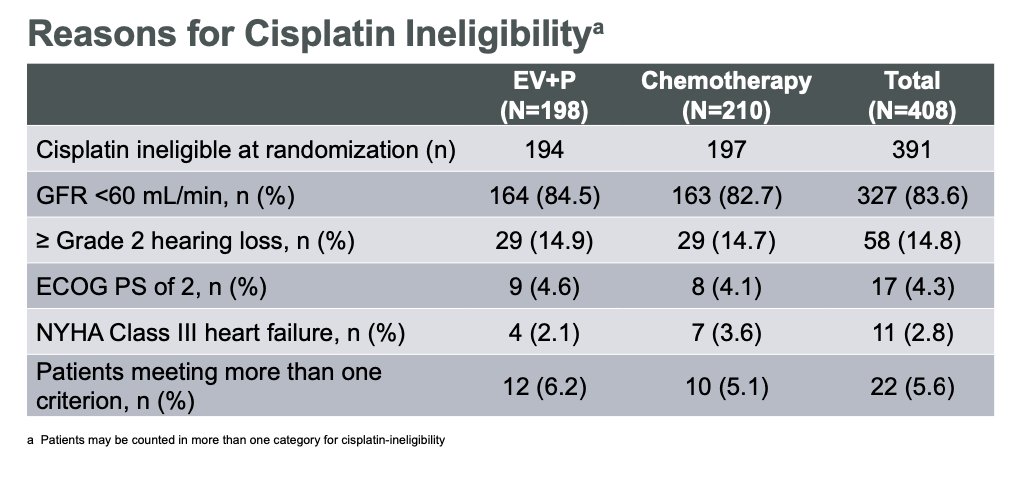
Importantly, Dr Van Der Heijden discussed the reasons for cisplatin ineligibility (See table below). Most patients were ineligible due to having a GFR <60 mL/min (83.6%). 205/210 (97.6%) of cisplatin-ineligible patients received carboplatin at Cycle 1 in the chemotherapy arm.
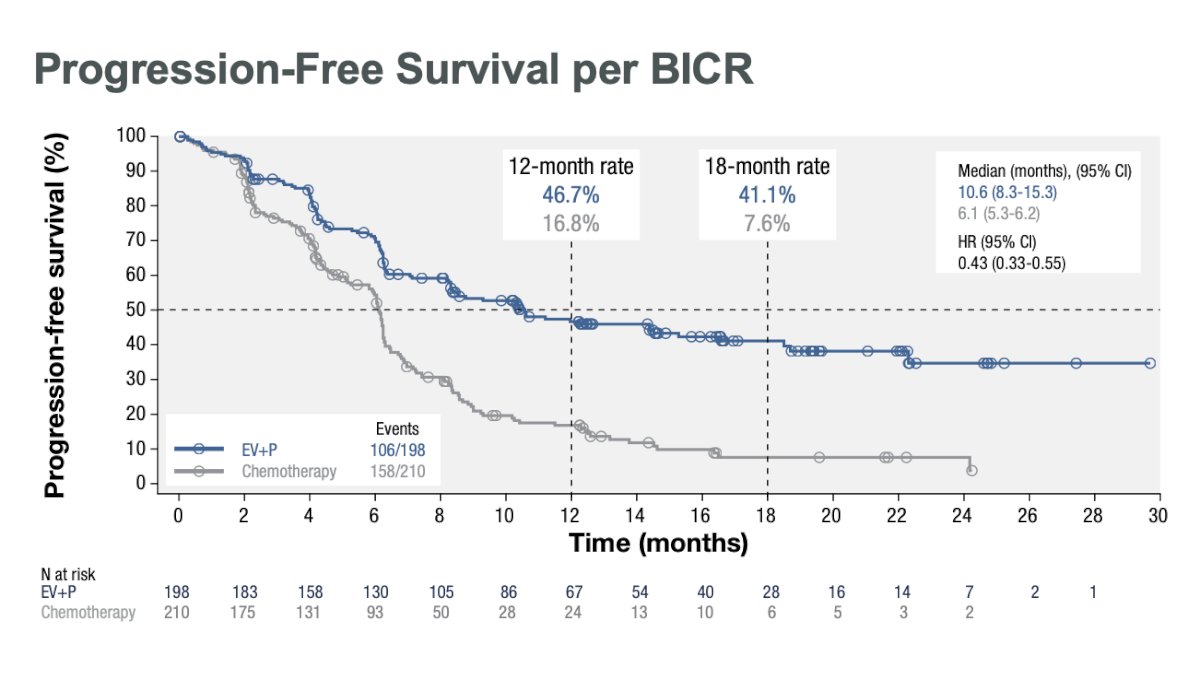
Compared to platinum-based chemotherapy, the combination of EV+P prolonged PFS from 6.1 to 10.6 months (HR: 0.43, 95% CI: 0.33 – 0.55, p<0.00001).
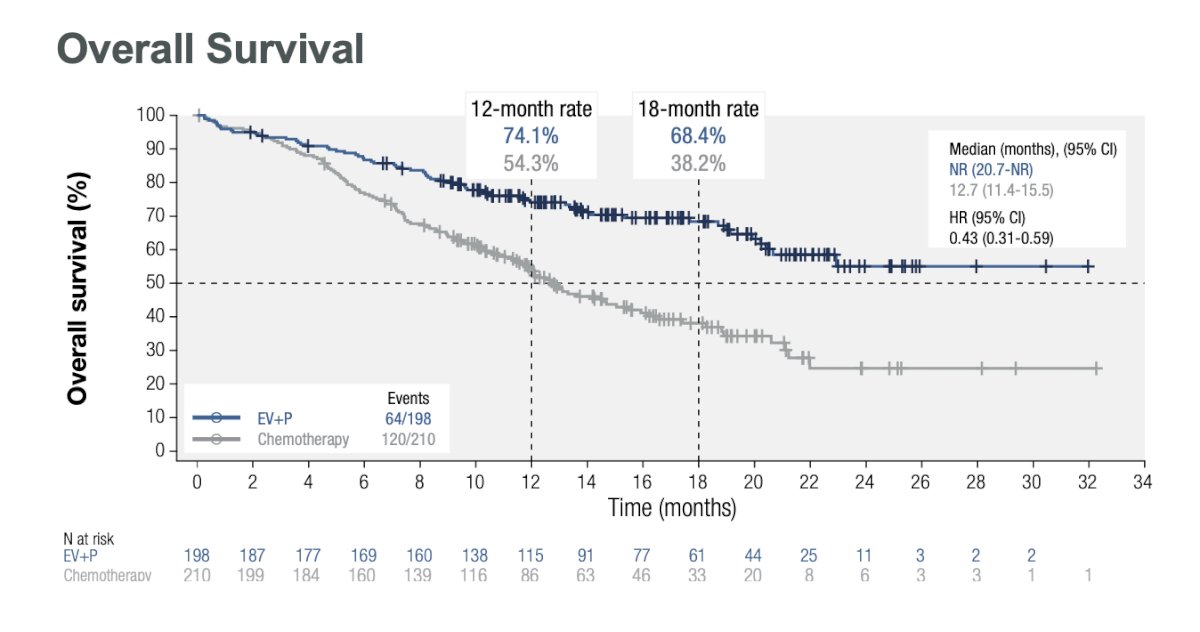
The overall survival was not reached in the cisplatin ineligible population treated with EV+P vs. median of 12.7 months (11.4-15.5) in the chemotherapy group (HR: 0.43, 95% CI: 0.31 – 0.59, p<0.00001).
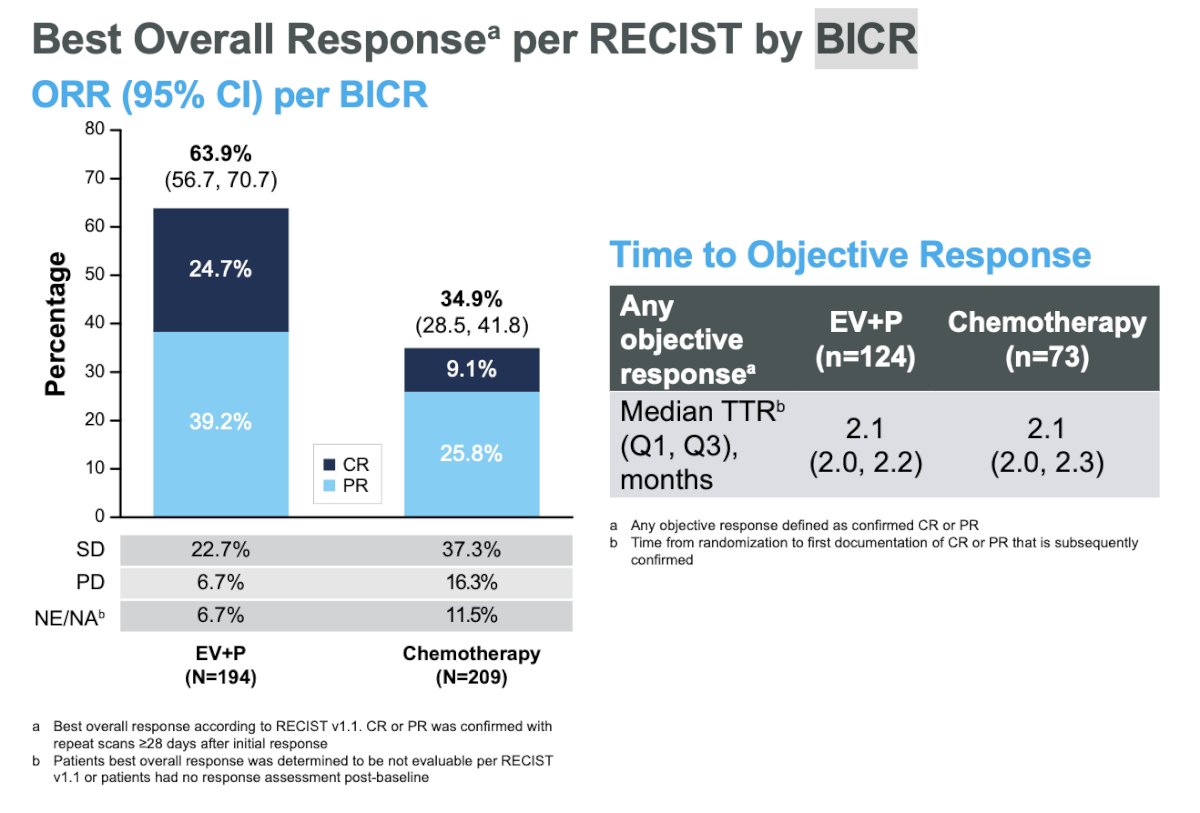
Objective response rate (ORR) was assessed via blinded independent central review per RECIST criteria. The ORR in the EV+P arm was 64% compared to 35% in the chemotherapy arm. The complete response (CR) rate in the EV+P arm was 25% vs. 9% in the chemotherapy arm.
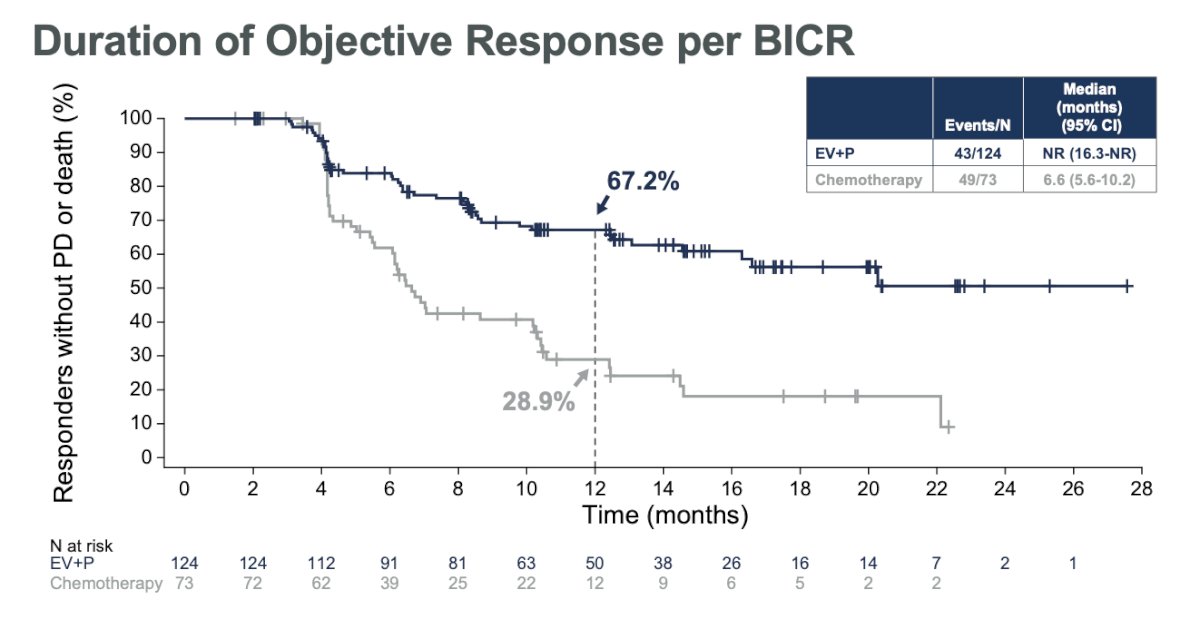
The median duration of ORR was not reached in the EV+P group compared to 6.6 months (5.6-10.2) in the chemotherapy group.
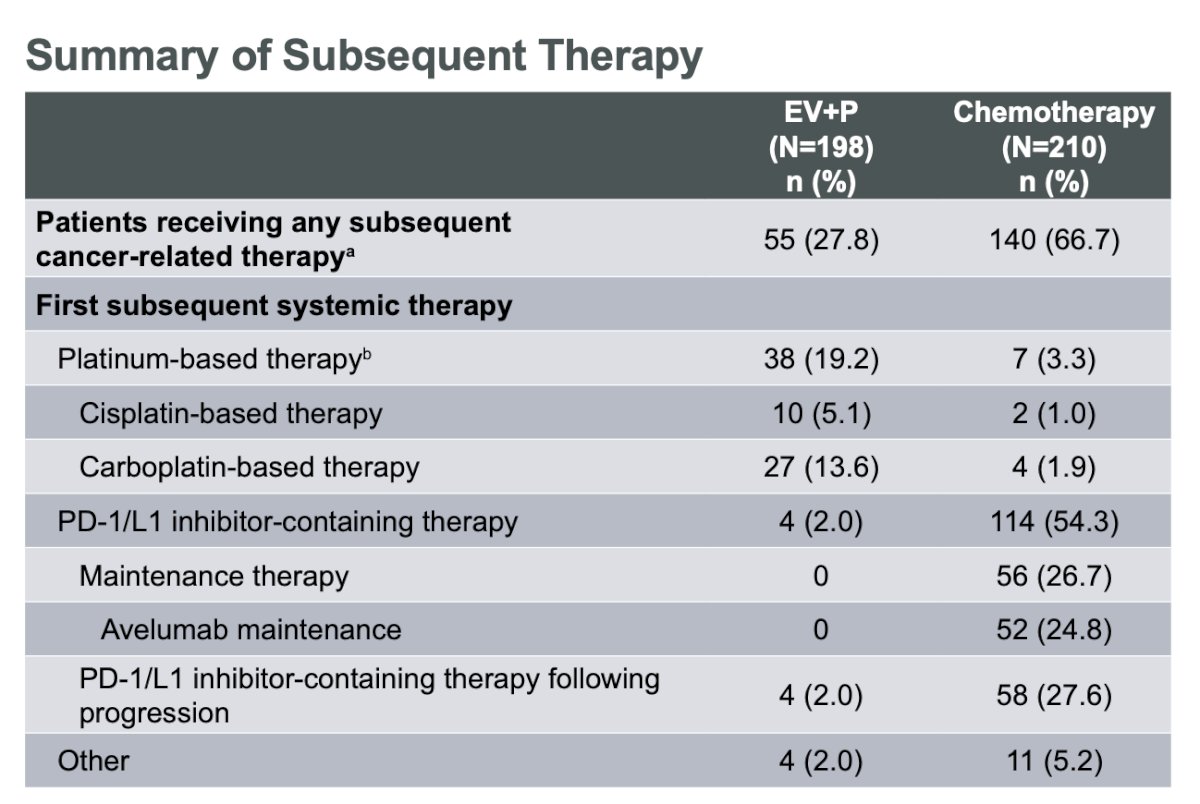
Of note, 26.7% of patients receiving chemotherapy received maintenance therapy; most of these patients received avelumab.
With regards to safety outcomes, the combination of EV+P was generally manageable with no new safety signals observed. Grade ≥3 events were observed in 58.4% of patients in the experimental arm, compared to 77% in the chemotherapy arm. Investigator-assessed, treatment-related adverse events leading to death occurred in 2 (1%) patients in the EV+P arm (diarrhea, multiple organ dysfunction syndrome) and in 3 (1.5% in the chemotherapy arm (febrile neutropenia, neutropenic sepsis, sepsis).
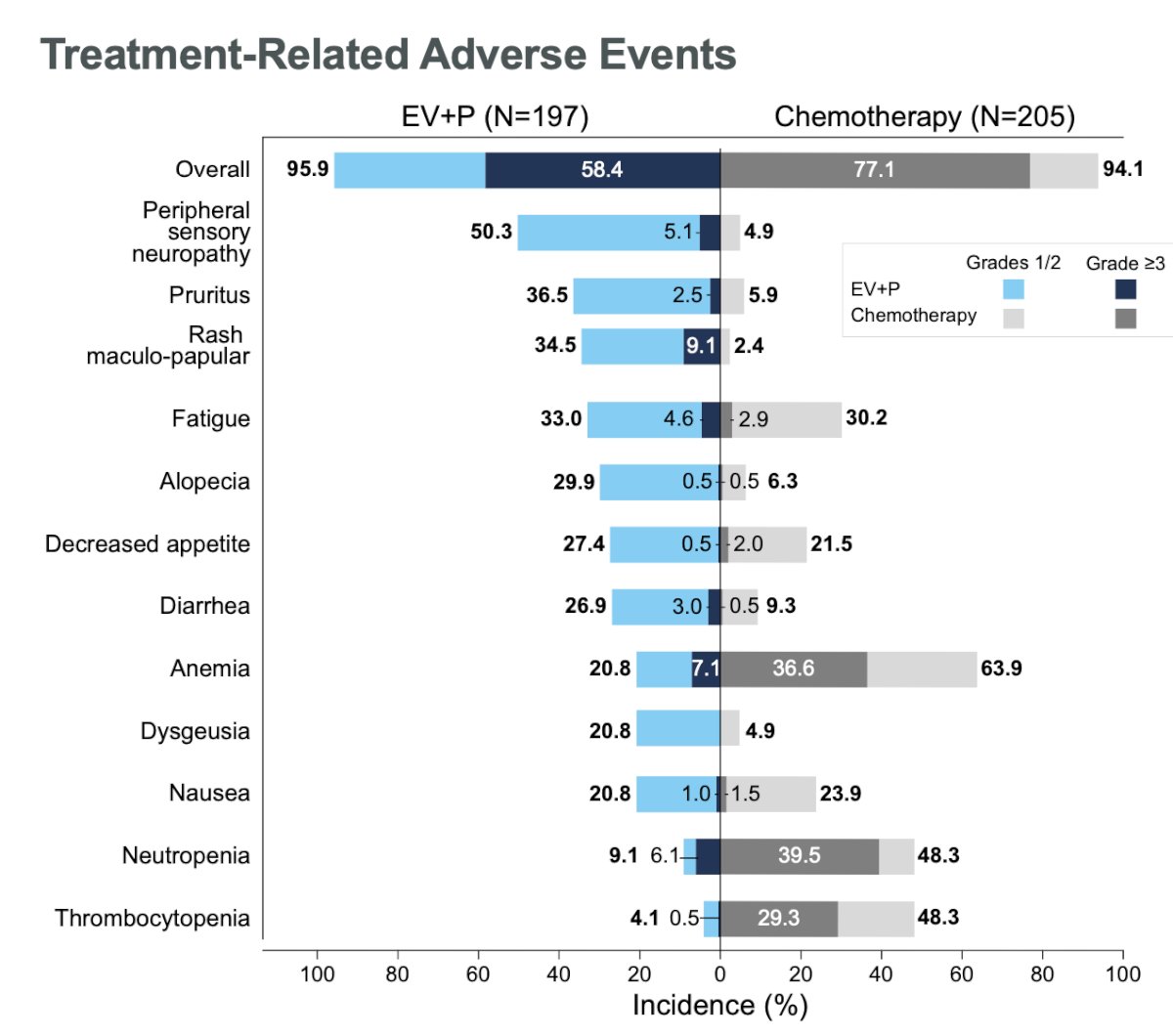
Dr. van der Heijden concluded that:
- Enfortumab vedotin + Pembrolizumab significantly improved progression-free and overall survival over chemotherapy in patients who were ineligible for cisplatin in the first line setting of la/mUC
- In the cisplatin ineligible patient population with historically poor prognosis, the risk of death was reduced by 57%
- The efficacy results of EV+P for the cisplatin-ineligible population were consistent with those of the overall population
- The safety profile of EV+P was generally manageable with no new safety signals in this patient population
- While EV+P is FDA approved for all-comers, the results of this analysis continue to support EV+P as a new SOC for la/mUC, including patients who are deemed ineligible to receive cisplatin.
Presented by: Michiel Simon Van Der Heijden, MD, PhD, Department of Medical Oncology, Netherlands Cancer Institute, Amsterdam, The Netherlands
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Related content: EV-302 Subgroup Analysis: Improved Outcomes for Cisplatin-Ineligible Advanced Urothelial Cancer Patients - Michiel Van der Heijden
References:
- Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012 Apr 1;30(10):1107-1113.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Powles T, Park SH, Caserta C, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After >/=2 Years of Follow-Up. J Clin Oncol. 2023;41: 3486-3492.
- van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023;389(19):1778-1789.
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.


