(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on bladder cancer, and a presentation by Dr. Roger Li discussing the final results of CORE-001, a phase-2, single arm study of cretostimogene grenadenorepvec in combination with pembrolizumab in patients with BCG-unresponsive, non-muscle invasive bladder cancer with CIS. Cretostimogene grenadenorepvec is a type-5 oncolytic adenovirus designed to selectively replicate in bladder cancer cells with alterations in the retinoblastoma pathway. Additionally, the virus is engineered to express the GM-CSF transgene, resulting in a potent oncolytic immunotherapy mode of action:
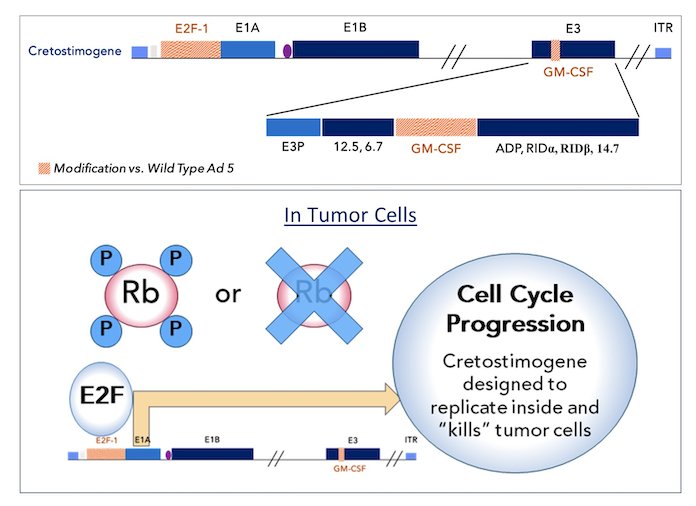
Cretostimogene monotherapy recently received FDA Fast Track and Breakthrough Therapy Designations in the BCG-unresponsive, high-risk, non-muscle invasive bladder cancer with carcinoma in situ indication with a complete response at any time rate of 76%. Intravenous pembrolizumab is FDA approved in BCG unresponsive non muscle invasive bladder cancer with a 41% complete response rate at any time and a 12 month complete response rate of ~20%.1 This phase-2 study assessed the potential synergy between intravesical cretostimogene and pembrolizumab in patients with BCG-unresponsive, high-risk, non-muscle invasive bladder cancer with carcinoma in situ, with or without Ta/T1 tumors. This combination has also received Breakthrough Therapy Designation from the FDA.
There were 35 patients treated with cretostimogene (1x1012 viral particles) in combination with pembrolizumab at a dose of 400 mg IV every 6 weeks. Cretostimogene induction was given as 6 weekly intravesical instillations followed by 3 weekly maintenance doses at months 3, 6, 9, 12, and 18. Patients with persistent CIS or high-grade Ta tumors at the 3 month assessment were eligible for re-induction:
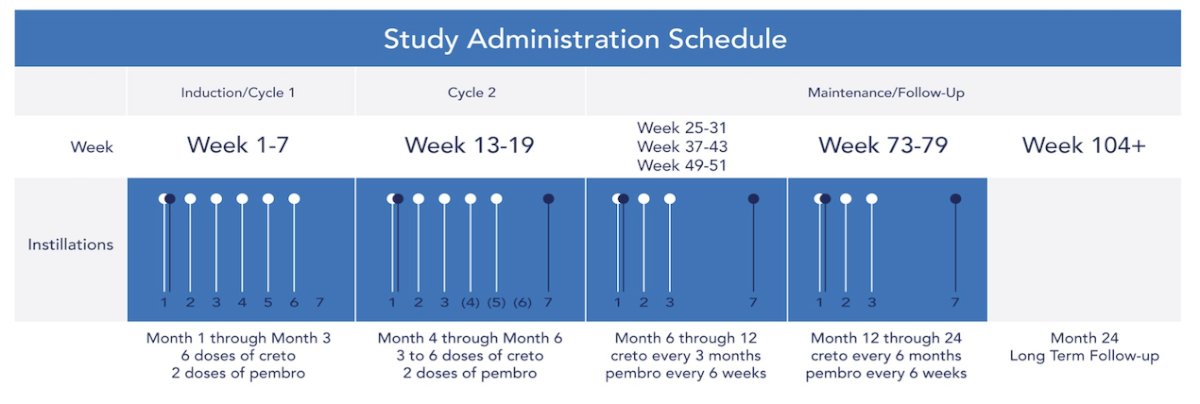
Pembrolizumab was administered for up to 24 months. Response assessments included cystoscopy, urine cytology, cross-sectional imaging, and mandatory bladder mapping biopsies at 12 months. The primary endpoint was complete response at 12 months. Secondary endpoints included complete response at any time, duration of response, complete response at 24 months, cystectomy-free survival, and safety. Exploratory endpoints included analyses of baseline viral receptor expression, free E2F levels, PD-L1 status, urinary cytokine panels, and measures of viral replication.
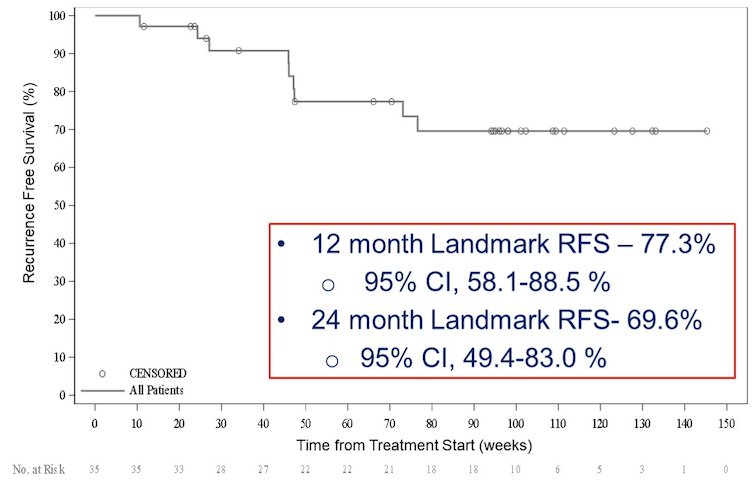
There were 30/35 patients evaluable per protocol for the primary endpoint. Five patients discontinued prior to the 12 month time point. The complete response rate in the intention to treat population at 12 months and any time, was 57.1% (20/35) (95% CI 39.5-73.2%) and 82.9% (29/35), respectively. The current complete response rate in the intention to treat population at 24 months is 54.3% (19/35) (95% CI 36.9-70.8%). Importantly, 95.1% of complete responses at 12 months remain in complete response at 24 months. The 12 month landmark recurrence free survival is 77.3% (95% CI 58.1%-88.5%) and the 24 month landmark recurrence free survival is 69.6% (95% CI 49.4-83.0%):
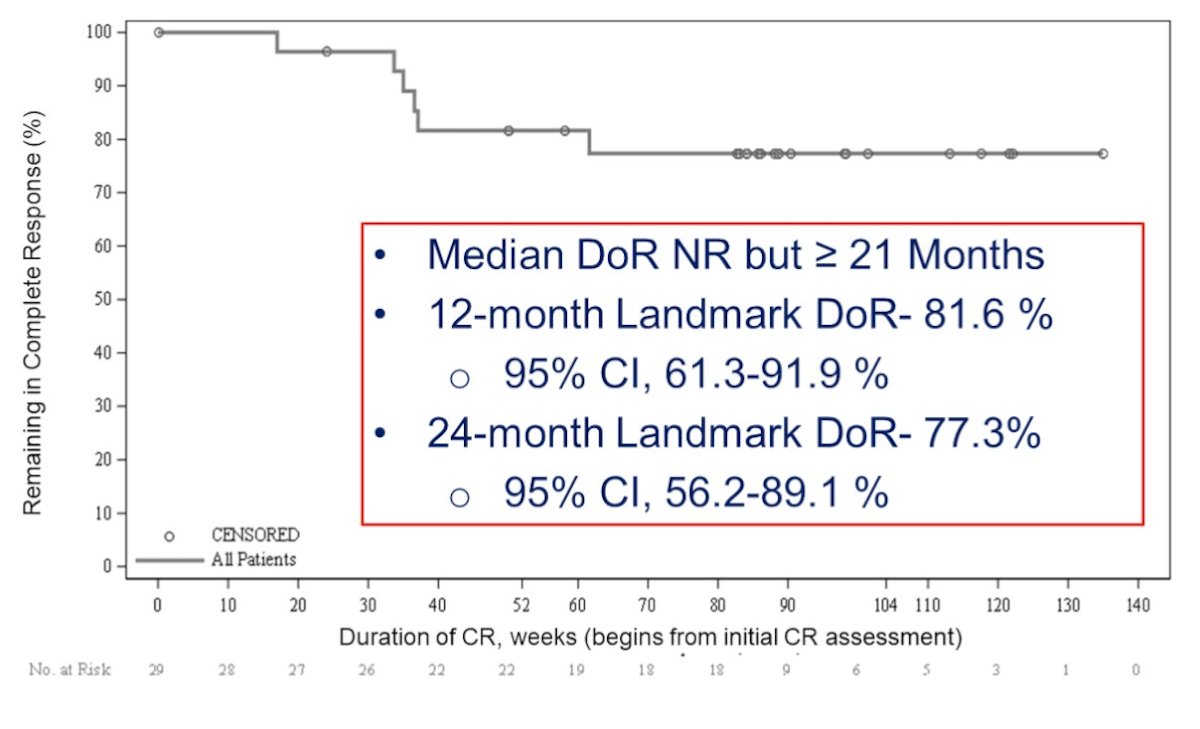
There was 100% progression free survival and no progression to muscle invasive or metastatic disease. Dr. Li notes that these results compare favorably to nadofaragene (94%), pembrolizumab (91%), and N803 + BCG (90%). The median duration of response has not been reached but exceeds 21 months. The 12 month landmark duration of response is 81.6% (95% CI 61.3-91.9%) and the 24 month landmark duration of response is 77.3% (95% CI 56.2-89.1%):
Analyses of treatment-related adverse events are consistent with the individual agents and demonstrate no synergistic toxicity.
Dr. Li concluded his presentation by discussing the final results of CORE-001 with the following take home messages:
- The efficacy and safety of cretostimogene plus pembrolizumab for treatment of BCG-unresponsive, high-risk, non-muscle invasive bladder cancer with carcinoma in situ demonstrates best-in-class complete response and duration of response compared to current FDA-approved therapies, with an acceptable adverse event profile
- Further investigation of this promising combination therapy is warranted and may serve to address a considerable unmet need
Presented by: Roger Li, MD, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:


