(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL was host to the Poster Session: Genitourinary Cancer: Kidney and Bladder. Dr. Joaquim Bellmunt presented the long-term outcomes from JAVELIN Bladder 100 in patients with low tumor burden treated with Avelumab first-line maintenance for advanced urothelial carcinoma (aUC).
In the study JAVELIN Bladder 100, avelumab first-line maintenance and best standard of care (BSC) significantly prolonged overall survival (OS) and progression-free survival (PFS) vs BSC alone in patients with advanced UC that had not progressed following first line platinum-based chemotherapy.1 The study design is illustrated below:
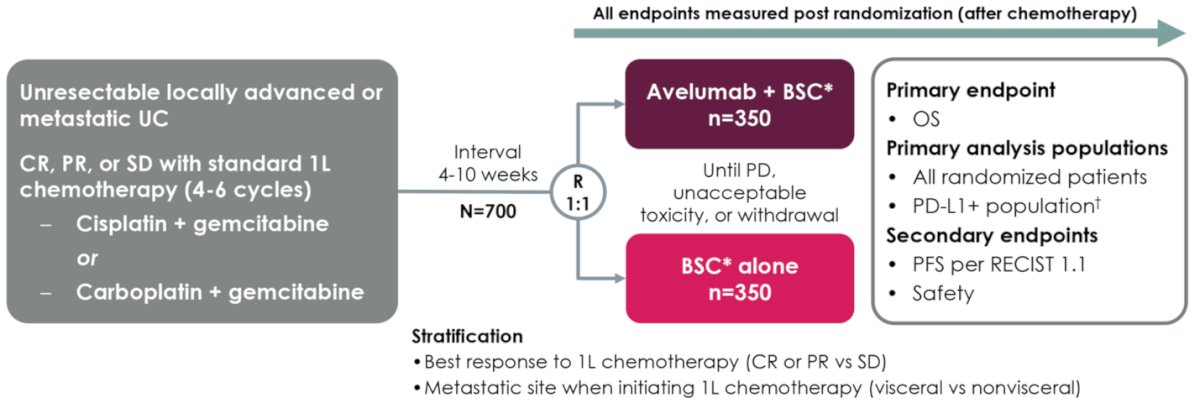
Prior analyses have shown that low tumor burden in patients with advanced UC is associated with better oncological outcomes when treated with immune checkpoint inhibitors2,3 Dr. Bellmunt presented a post hoc analysis of efficacy and safety of JAVELIN Bladder 100 in subsets of patients with low tumor burden. Patients with low tumor burden had either nonvisceral metastases at start of chemotherapy or lymph node–only disease at randomization.
At the efficacy data cutoff (June 4, 2021), the median follow-up was ≥38 months in both arms. In all subgroups of patients with low tumor burden, OS was prolonged in the avelumab + BSC arm vs the BSC alone arm:
- Nonvisceral metastasis: median OS 31 months vs. 17 months
- Lymph node-only disease: median OS 32 months vs. 23 months
- Pelvic/retroperitoneal nodal disease: median OS 31 months vs. 20 months
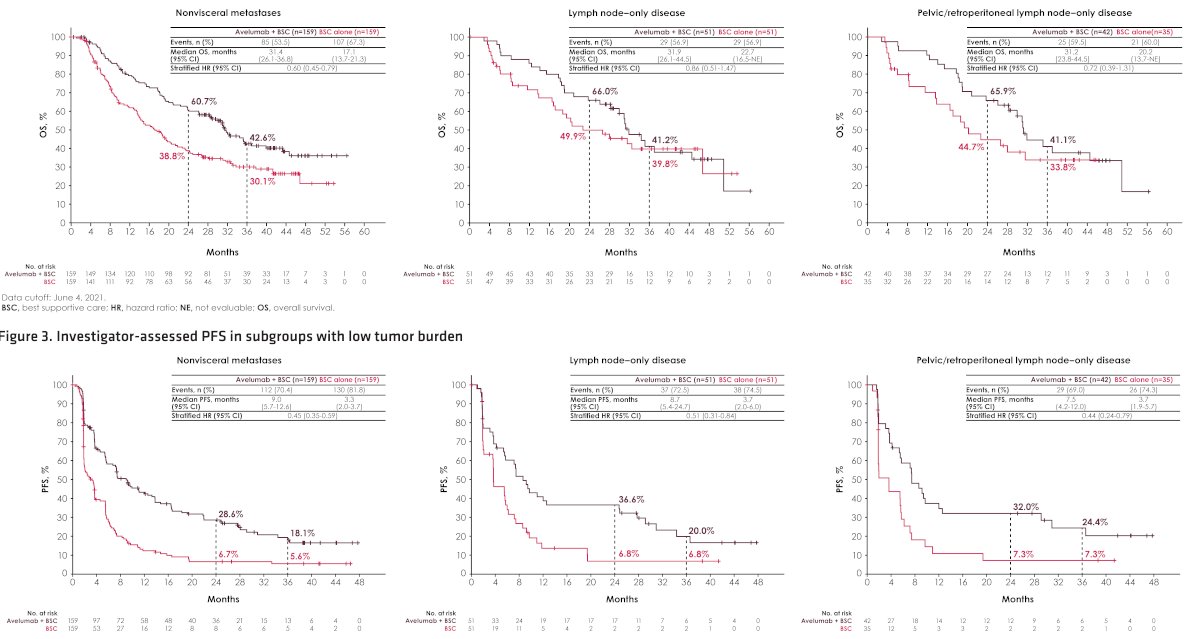
Compared to BSC the combination of BSC + Avelumab prolonged progression-free survival in all subgroups of patients with low tumor burden.

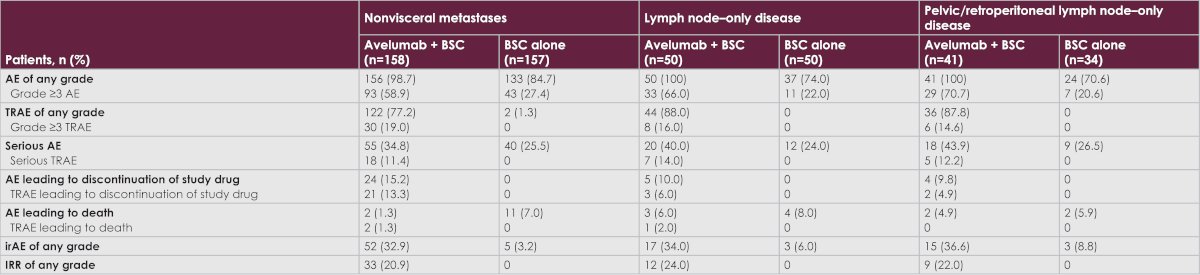
With regards to safety outcomes, the long-term safety of avelumab 1L maintenance was acceptable in all subsets of patients with low tumor burden, toxicity was generally manageable with no new safety signals observed in the low tumor burden population. Grade ≥3 events were observed in 19% of patients in the experimental arm, compared to 0% in the BSC arm. Treatment related adverse events leading to death occurred in two patients (1.3%).
Dr. Bellmunt concluded that:
- In this post hoc analysis of JAVELIN Bladder 100 trial, OS and PFS were prolonged with avelumab + BSC vs BSC alone in subgroups of patients with low tumor burden
- The long-term safety of avelumab first line maintenance in patients with low tumor burden advanced UC was demonstrated and was consistent with results from the overall population
- These findings indicate that avelumab 1L maintenance has pronounced efficacy and manageable toxicity in patients with advanced UC with low tumor burden supporting the use of platinum-based chemotherapy followed by avelumab maintenance as an important 1L treatment option in these patients
Presented by: Joaquim Bellmunt, MD, PhD, Associate Professor at Harvard Medical School and Director of Bladder Cancer Center at Genitourinary Oncology Program of Dana-Farber Cancer Institute. Boston, Massachusetts.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:- Powles T, Sridhar SS, Loriot Y, Bellmunt J, Mu XJ, Ching KA, Pu J, Sternberg CN, Petrylak DP, Tambaro R, Dourthe LM, Alvarez-Fernandez C, Aarts M, di Pietro A, Grivas P, Davis CB. Avelumab maintenance in advanced urothelial carcinoma: biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat Med. 2021 Dec;27(12):2200-2211. doi: 10.1038/s41591-021-01579-0. Epub 2021 Dec 10. PMID: 34893775.
- Vuky J, Balar AV, Castellano D, O'Donnell PH, Grivas P, Bellmunt J, Powles T, Bajorin D, Hahn NM, Savage MJ, Fang X, Godwin JL, Frenkl TL, Homet Moreno B, de Wit R, Plimack ER. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol. 2020 Aug 10;38(23):2658-2666. doi: 10.1200/JCO.19.01213. Epub 2020 Jun 17. PMID: 32552471.
- Nassar AH, Mouw KW, Jegede O, Shinagare AB, Kim J, Liu CJ, Pomerantz M, Harshman LC, Van Allen EM, Wei XX, McGregor B, Choudhury AD, Preston MA, Dong F, Signoretti S, Lindeman NI, Bellmunt J, Choueiri TK, Sonpavde G, Kwiatkowski DJ. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br J Cancer. 2020 Feb;122(4):555-563. doi: 10.1038/s41416-019-0686-0. Epub 2019 Dec 20. PMID: 31857723; PMCID: PMC7028947.


