(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL was host to the Poster Session: Genitourinary Cancer: Kidney and Bladder. Dr. Richard Bryce presented the trial in progress LEGEND study: a phase 1/2 study of EG-70 (detalimogene voraplasmid) intravesical monotherapy for patients with BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ (NCT04752722).
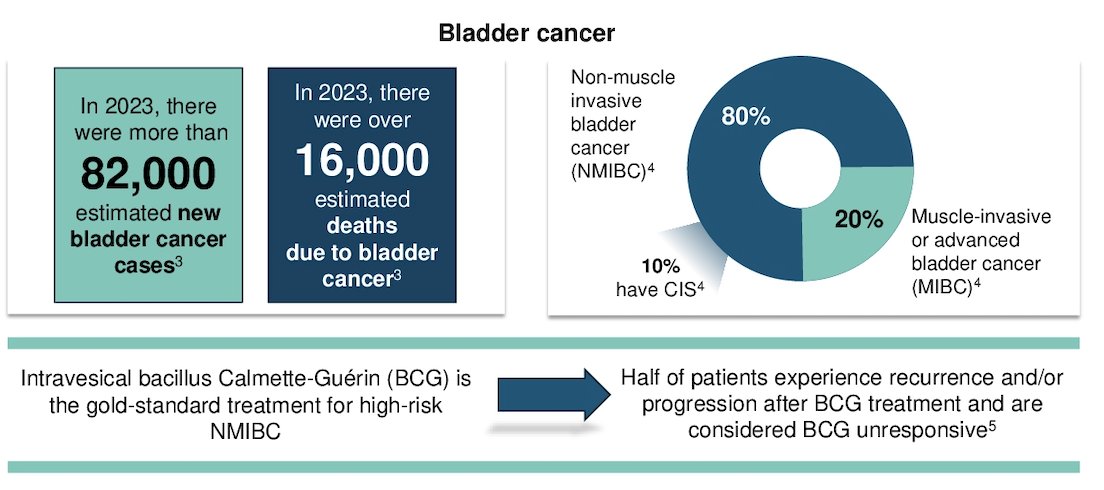
Dr. Bryce began his presentation by highlighting that bladder-sparing therapies for BCG-unresponsive non-muscle invasive bladder cancer are an important unmet need. In 2023 alone, 82,000 new bladder cancer cases were diagnosed in the United States, with 80% being non-muscle invasive cases.1 The standard of care for patients with high-risk disease is intravesical bacillus Calmette-Guerin (BCG). Unfortunately, ~50% of patients experience recurrence or progression after BCG treatment and are deemed to have BCG-unresponsive disease, for which radical cystectomy remains the standard of care.
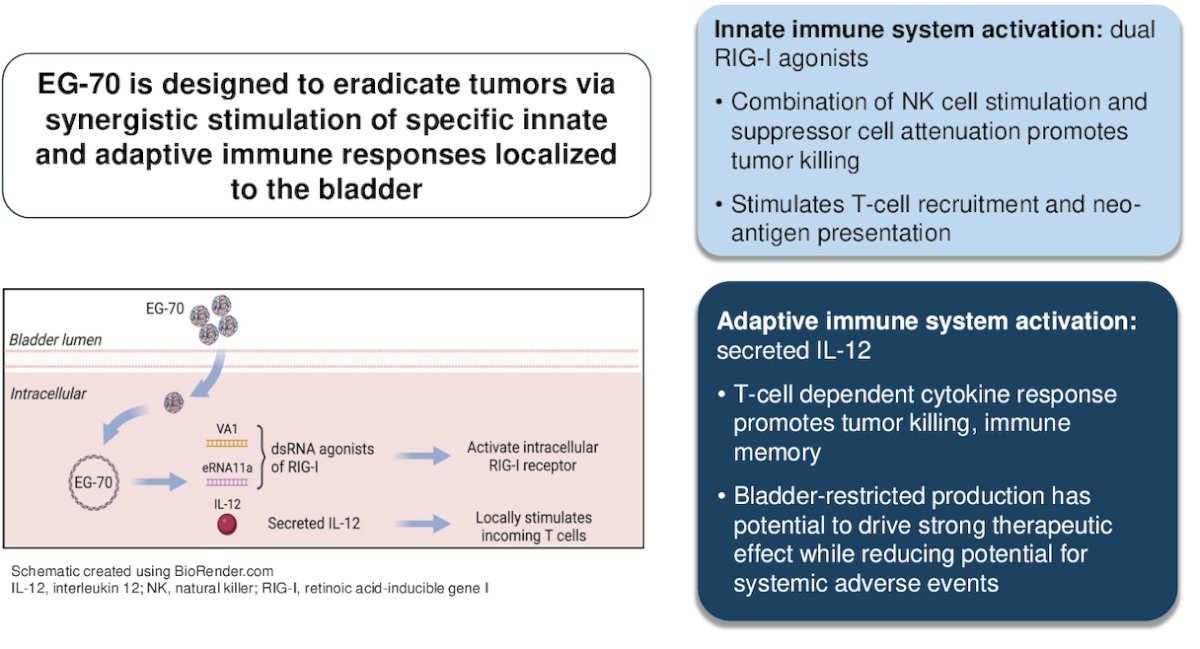
EG-70 (detalimogene voraplasmid) is an investigational, non-viral gene-based immunotherapy that carries a plasmid that simultaneously expresses a recombinant single-chain human interleukin-12 (IL-12) and two double-stranded RNA activators of the intracellular innate immunity regulator, retinoic acid-inducible gene I (RIG-I).
EG-70, by activation of the RIG-I, stimulates the innate immune system, leading to a combination of natural killer (NK) cell stimulation, T-cell recruitment, and suppressor cell attenuation. Moreover, adaptive immune system stimulation by expressing recombinant human IL-12 leads to a T-cell-dependent cytokine response, promoting tumor killing and the creation of immune memory. The mechanism of action of EG-70 is illustrated in the graphic below:
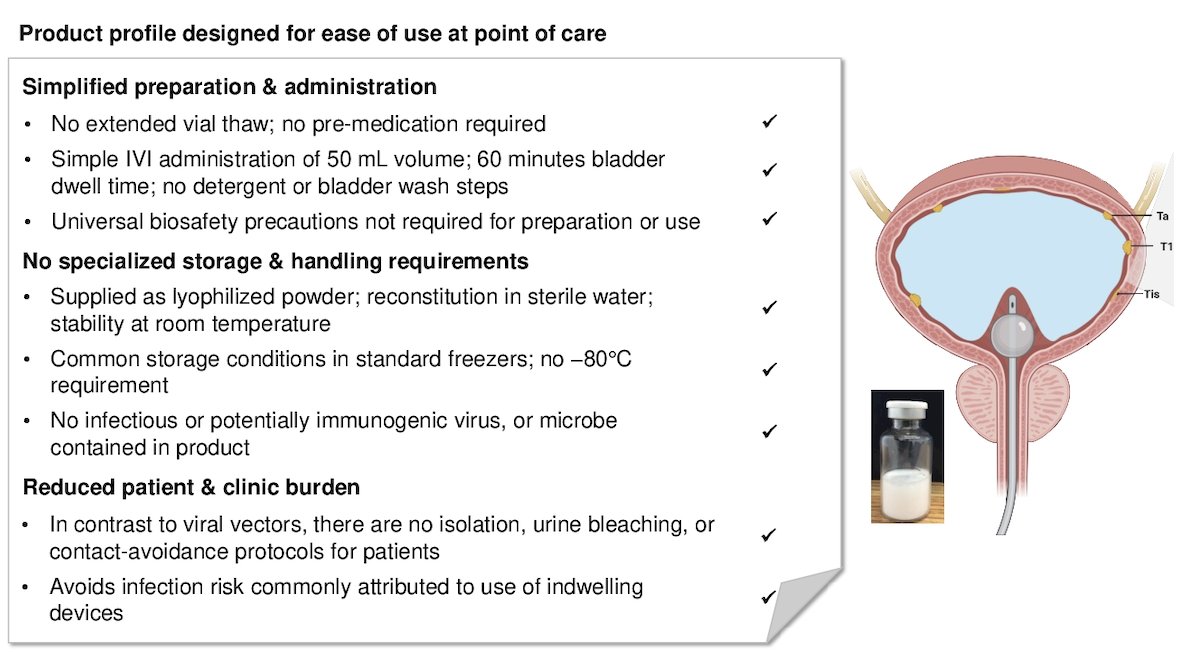
The EG-70 genetic medicine-based immunotherapy was specifically engineered for intravesical administration to elicit local stimulation of anti-tumor immune responses and drive durable efficacy in BCG-unresponsive non-muscle invasive bladder cancer, aiming to minimize the risk of systemic toxicity from intravesical therapy. Some advantages of EG-70 include simplified preparation and administration, no specialized storage or onerous handling requirements, and reduced patient and clinic burden since it does not require isolation or contact-avoidance protocols for patients. Additionally, it avoids the infection risk attributed to the use of indwelling catheters.
Findings from the Phase 1 dose-escalating portion of the LEGEND study (NCT04752722) were presented by Dr. Gordon Brown at the American Urological Association (AUA) 2024 Annual Meeting in San Antonio, Texas. In the Phase 1 part of the study, three escalating doses of EG-70 were assessed, administered on two schedules at study Weeks 1 and 2 to BCG-unresponsive patients with carcinoma in situ (CIS). Patients received either 2 or 4 doses over a 12-week period, following a 3+3 dose escalation design, with dose levels of 0.25 mg/mL, 0.8 mg/mL, and 2.5 mg/mL. After completing the 12-week study, patients showing either a complete response (CR) or stable disease (SD) were allowed to continue EG-70 for up to 3 additional 12-week cycles.2,3
In the 12-week Phase 1 trial segment, 24 patients were enrolled and received at least one dose of EG-70, totaling 175 doses administered. There were no safety concerns noted, and no dose-limiting toxicities were observed that necessitated dose de-escalation. In terms of efficacy, their secondary objective, 22 patients were evaluable for this outcome. A total of 15/22 (68%) experienced a CR at 3 months, with an additional 1/22 (5%) achieving CR at some point in treatment.
Dr Bryce presented the ongoing phase 2 portion of the LEGEND study, The study design is illustrated below.
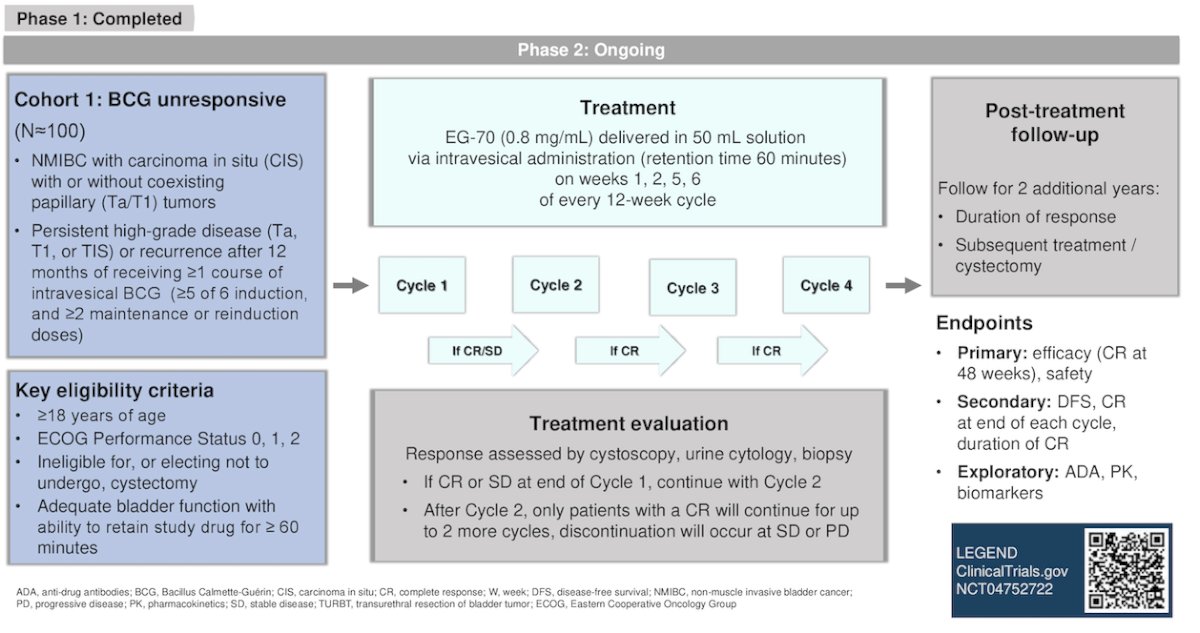
To summarize his presentation, Cohort 1 of the LEGEND phase 2 study aims to enroll 100 patients with carcinoma in situ +/- papillary tumors and patients with persistent/recurrent HG Ta, T1, or CIS after one or more courses of BCG (≥5 of 6 induction, and ≥2 maintenance or reinduction doses). The patients will be treated with intravesical EG-70 on weeks 1, 2, 5, and 6 of every 12-week cycle for a total of four cycles. The primary endpoint is the complete response rate at 48 weeks.
Presented by: Richard Bryce, MBChB, MRCGP, MFPM, Chief Medical Officer at enGene
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- SEER 2023. https://seer.cancer.gov/statfacts/html/urinb.html (Accessed March 19, 2024)
- Steinberg G, et al. SUO Annual Meeting, Washington, DC, November 28-December 1, 2023
- Kalota S, et al. AUA Annual Meeting, San Antonio, Texas, May 3-6, 2024.


