(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Poster Session: Genitourinary Cancer: Kidney and Bladder. Dr. Jens Bedke presented their poster titled: Enfortumab vedotin (EV) with pembrolizumab (P) versus chemotherapy in previously untreated locally advanced or metastatic urothelial carcinoma (la/mUC): Analysis of cisplatin (cis)-eligible population from EV-302/KEYNOTE-A39.
Dr. Bedke began by discussing that EV-302/KEYNOTE-A39 (NCT04223856) is a phase 3, randomized, open-label, global study comparing EV+P with platinum-based chemotherapy for first-line treatment of patients with locally advanced/metastatic urothelial carcinoma (la/mUC) regardless of cisplatin eligibility (1). In the EV-302 trial, EV+P demonstrated a significant clinically meaningful benefit compared with chemotherapy for the dual primary endpoints of progression-free survival (PFS) (hazard ratio [HR]: 0.45; P<0.00001) and overall survival (OS) (HR: 0.47; P<0.00001) in the overall patient population. This led the FDA to approve EV+P in December 2023 for the treatment of adults with la/mUC. For this presentation, the researchers focused on patients in the EV-302 trial who were eligible for cisplatin at randomization.
In EV-302, patients with previously untreated la/mUC were randomized 1:1 to receive 3-week cycles of EV (1.25 mg/kg IV; Days 1 and 8) and pembrolizumab (200 mg IV; Day 1) or platinum-based chemotherapy (gemcitabine with cisplatin or carboplatin). Patients were deemed eligible/ineligible for cisplatin by the protocol’s defined criteria:
- Glomerular filtration rate (GFR) of 30 to less than 60 ml per minute per 1.73 m2 of body-surface area.
- Hearing loss of grade 2 or higher
- Eastern Cooperative Oncology Group performance-status (ECOG-PS) score of 2
- New York Heart Association (NYHA) class III heart failure at enrollment
The study design is outlined in the figure below.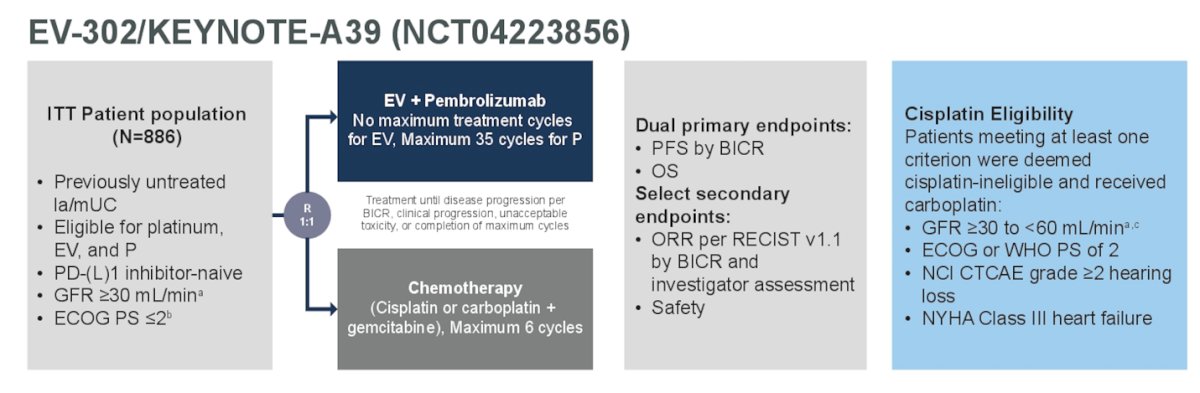
Dr. Bedke reported that a total of 478 patients were cisplatin-eligible at randomization, with 244 in the EV+P arm and 234 in the chemotherapy arm. Ninety-four percent of the patients in the chemotherapy arm received at least one cycle of platinum-based chemotherapy. Baseline characteristics were well balanced; however, he noted that liver metastasis was more pronounced in the EV + P arm (20.5% vs. 19.7%). The rest of the baseline characteristics are shown in the table below.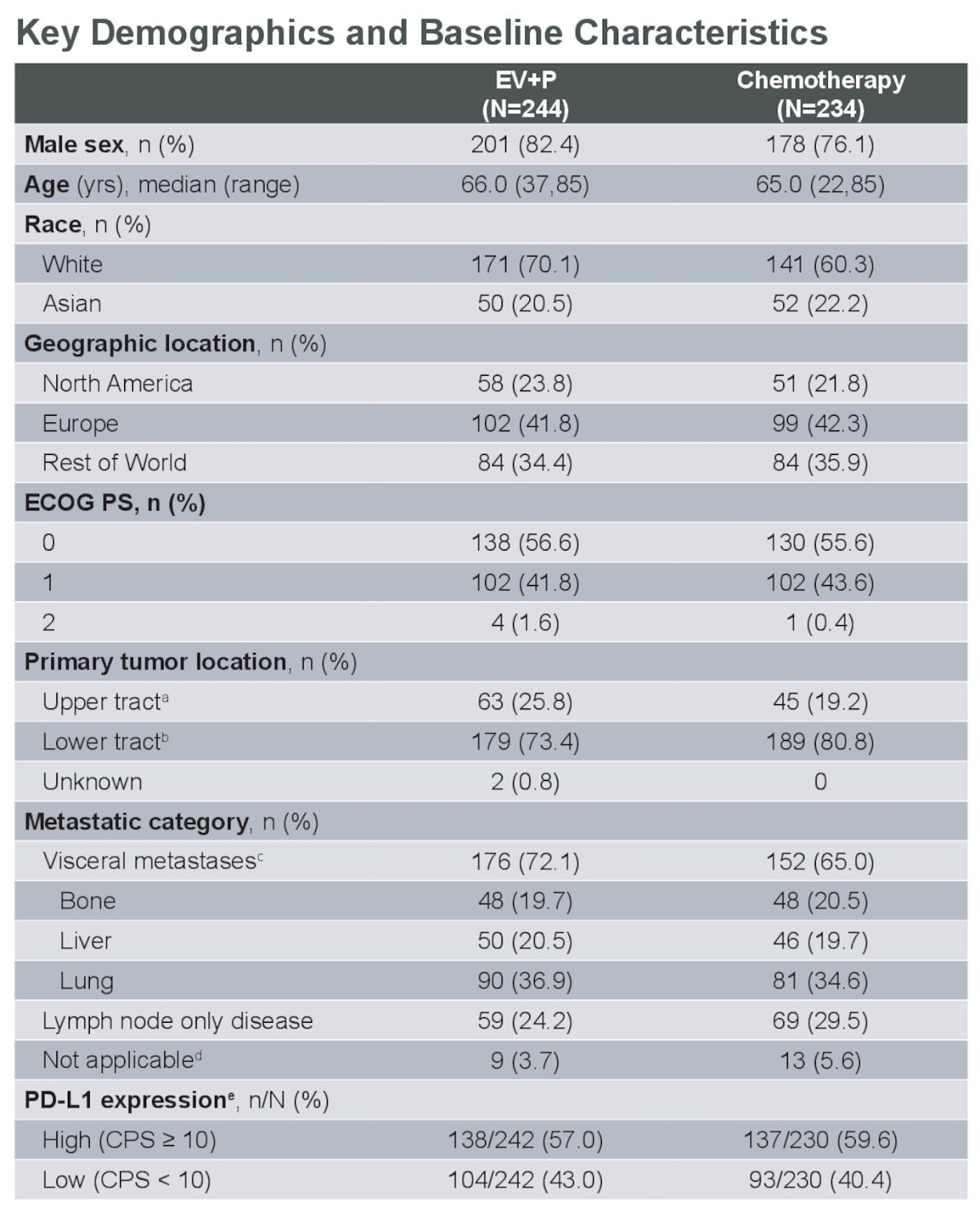
In the cis-eligible cohort, the median PFS was 14.6 months for EV+P compared to 6.5 months for chemotherapy, with a 52% reduction in the risk of progression (HR: 0.48, 95% CI: 0.38, 0.62).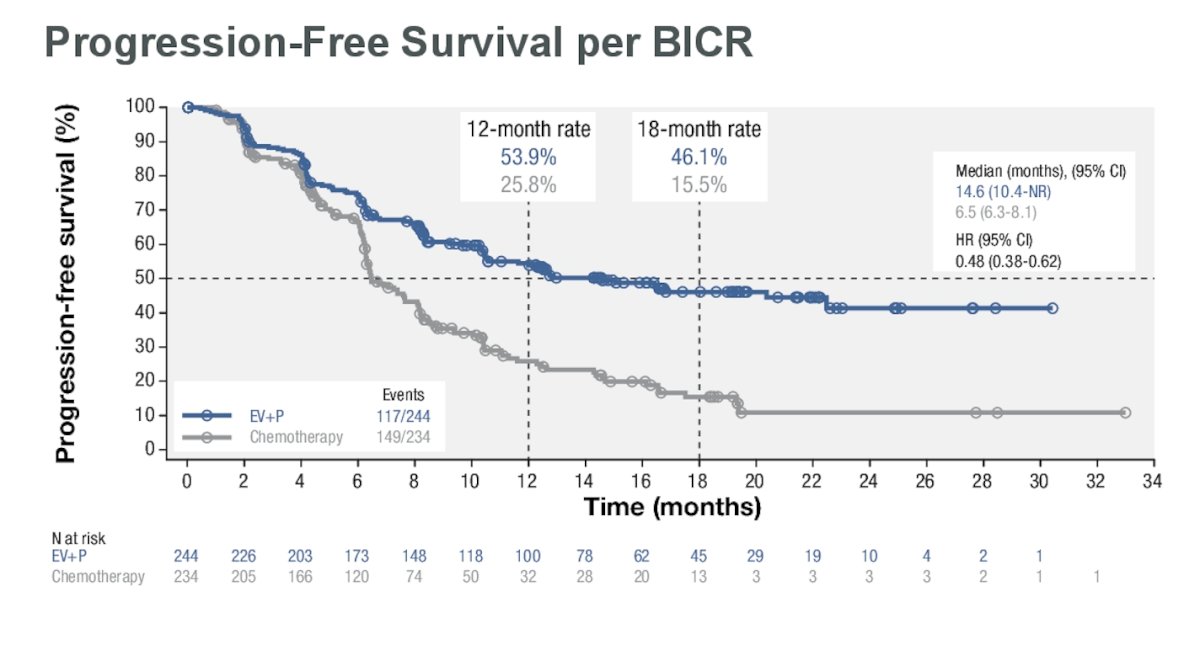
The median OS was 31.5 months for EV+P vs. 18.4 months for chemotherapy, showing a 47% reduction in the risk of death (HR: 0.53, 95% CI: 0.39, 0.72).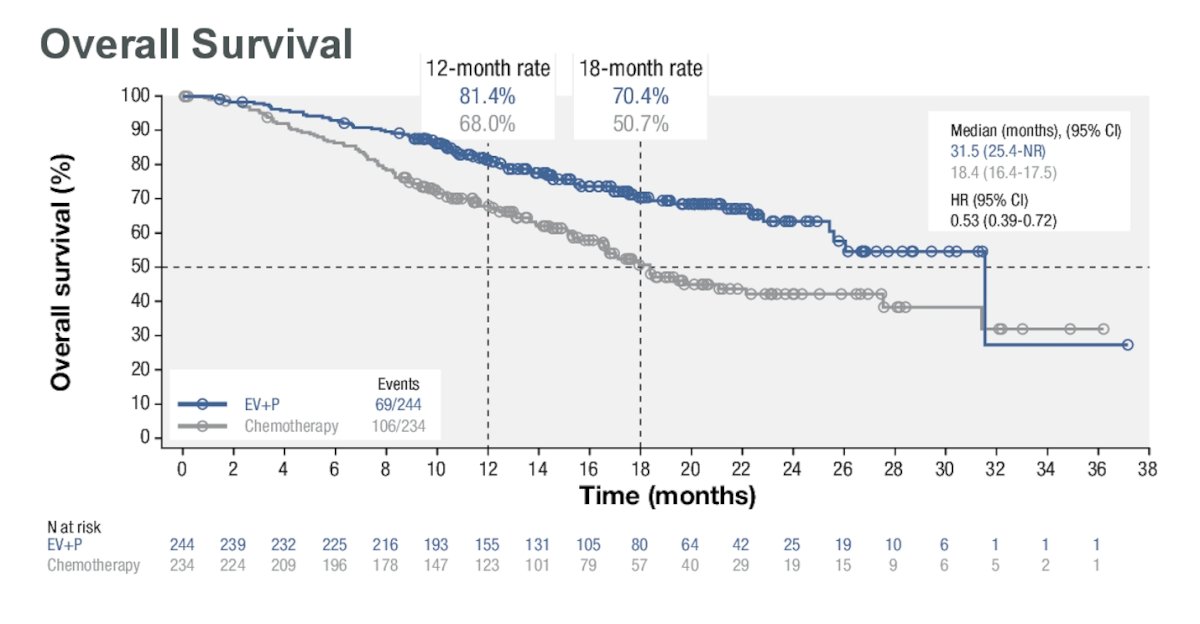
For the cis-eligible population, the overall response rate (ORR) for EV+P was 70.8%, with 32.5% achieving a complete response. The ORR for chemotherapy was 53%, with a 15.5% complete response rate. The time to OR was similar between both arms (median 2.1 months).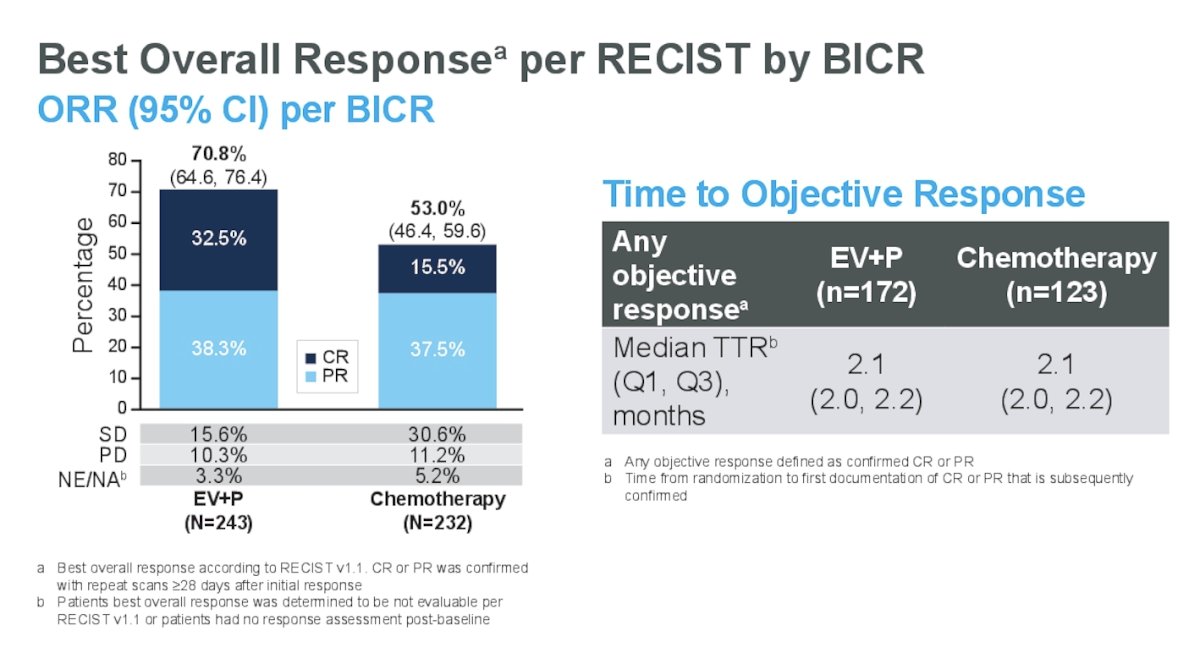
For the EV+P arm, the median duration of response was not reached (95% CI: 18.2 months, NR) compared to a median duration of response for the chemotherapy arm of 8.3 months (95% CI: 5.9, 10.9).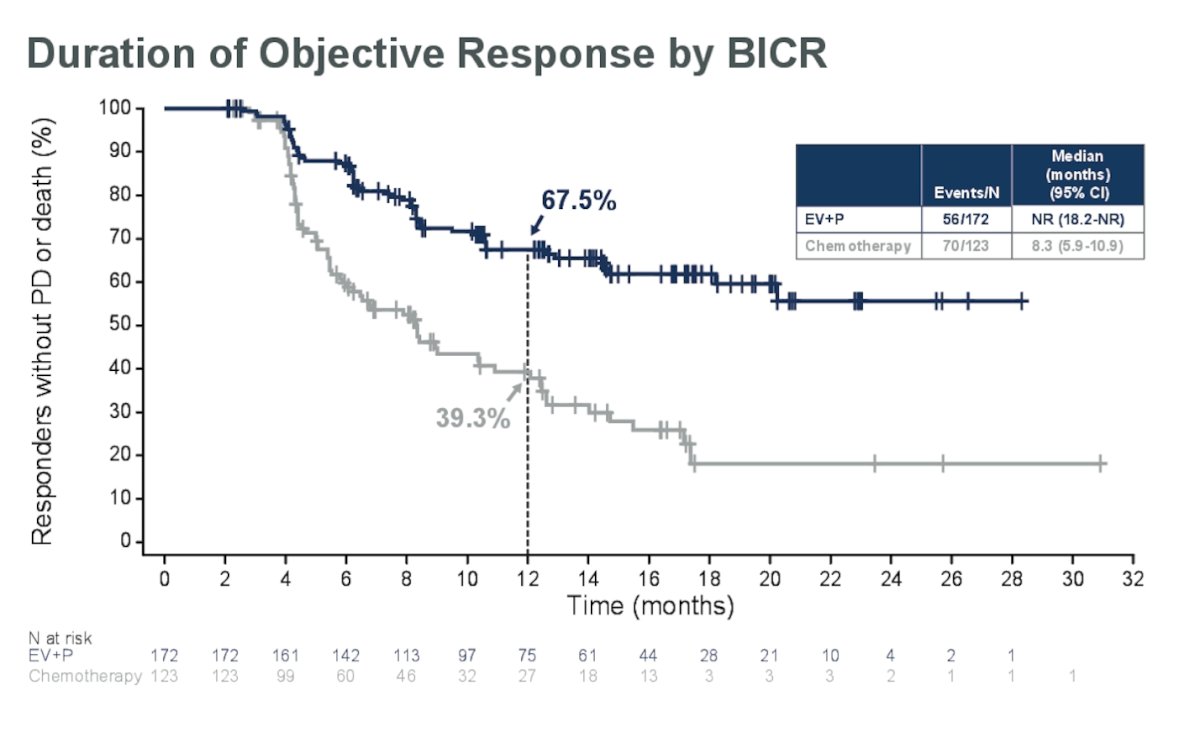
In the cis-eligible population, 34% of patients in the EV+P arm remained on treatment at the data cutoff, and 34.8% received subsequent therapy. Seventy-two patients (29.5%) received platinum-based therapy as their first subsequent therapy, with 17.6% receiving cisplatin. In the chemotherapy arm, all patients were off-study treatment at the time of data cutoff. 62.4% received any program death-1 or program death ligand-1 (PD-1/L1) therapy following chemotherapy. Eighty-three patients received maintenance avelumab, and 59 patients received PD-1/L1 therapy as second-line treatment.
Treatment-related adverse events (TRAEs) Grade ≥3 occurred in 53.9% of patients in the EV+P arm and 62.7% of patients in the chemotherapy arm in the cis-eligible population. The most common grade ≥3 TRAEs of special interest for EV were skin reactions (14.8%), hyperglycemia (7.8%), and peripheral neuropathy (5.8%). The most common grade ≥3 treatment-emergent adverse events of special interest for platinum-based chemotherapy were severe skin reactions (9.5%), pneumonitis (4.9%), and colitis (2.9%). TRAEs are depicted in the figure below.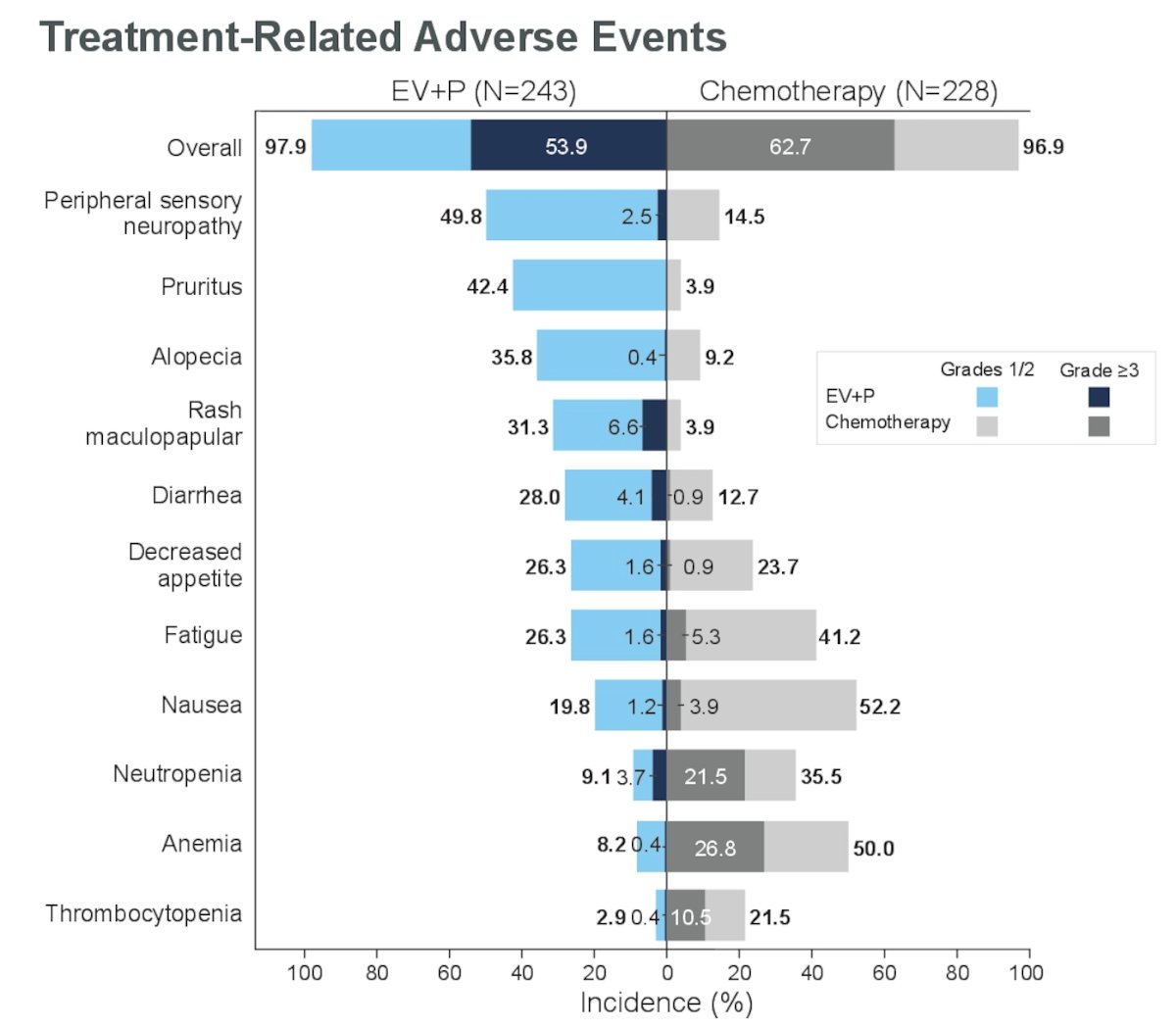
Dr. Bedke wrapped up his presentation concluding that in the cisplatinum-eligible population of the EV-302/KEYNOTE-A39 trial:
- EV+P improved clinical outcomes, reducing the risk of death by 47% and the risk of progression by 52% compared with platinum-based chemotherapy.
- EV+P showed a significant ORR of 70.8%, with 32.5% of patients achieving a complete response
- The Results of this cisplatin-eligible population analysis were consistent with the overall population.
- The results of EV-302 support EV+P as a new SOC for la/mUC, including patients who are eligible for cisplatin.
Presented by: Jens Bedke, MD, Professor, Urologic Oncology, Vice-Chairman of the Department of Urology, University of Tubingen, Germany.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
Reference:


