(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a kidney and bladder trials-in-progress poster session Dr. Matt Galsky presented the DV-001 trial, an ongoing phase 3 study of disitamab vedotin plus pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial carcinoma (la/mUC) expressing HER2.
Platinum-based chemotherapy has long been the standard 1st line therapy for la/mUC, which is a known aggressive disease. Recently, enfortumab vedotin, a Nectin-4-directed antibody-drug conjugate (ADC) with a Monomethyl auristatin E (MMAE) payload, combined with pembrolizumab has shown improved outcomes over chemotherapy.1 Novel biomarker-informed strategies may further improve outcomes.
HER2 expression (defined as IHC ≥1+) has been reported in approximately half of all patients across multiple tumor types, including urothelial carcinoma, and may be associated with poor outcomes. Disitamab vedotin (DV; RC48-ADC) is an investigational ADC comprising a fully humanized HER2-directed monoclonal antibody, disitamab, conjugated to MMAE via a protease-cleavable mc-vc linker. DV elicits antitumor activity through multimodal mechanisms of action, including MMAE-mediated direct cytotoxicity, bystander effect, and immunogenic cell death.
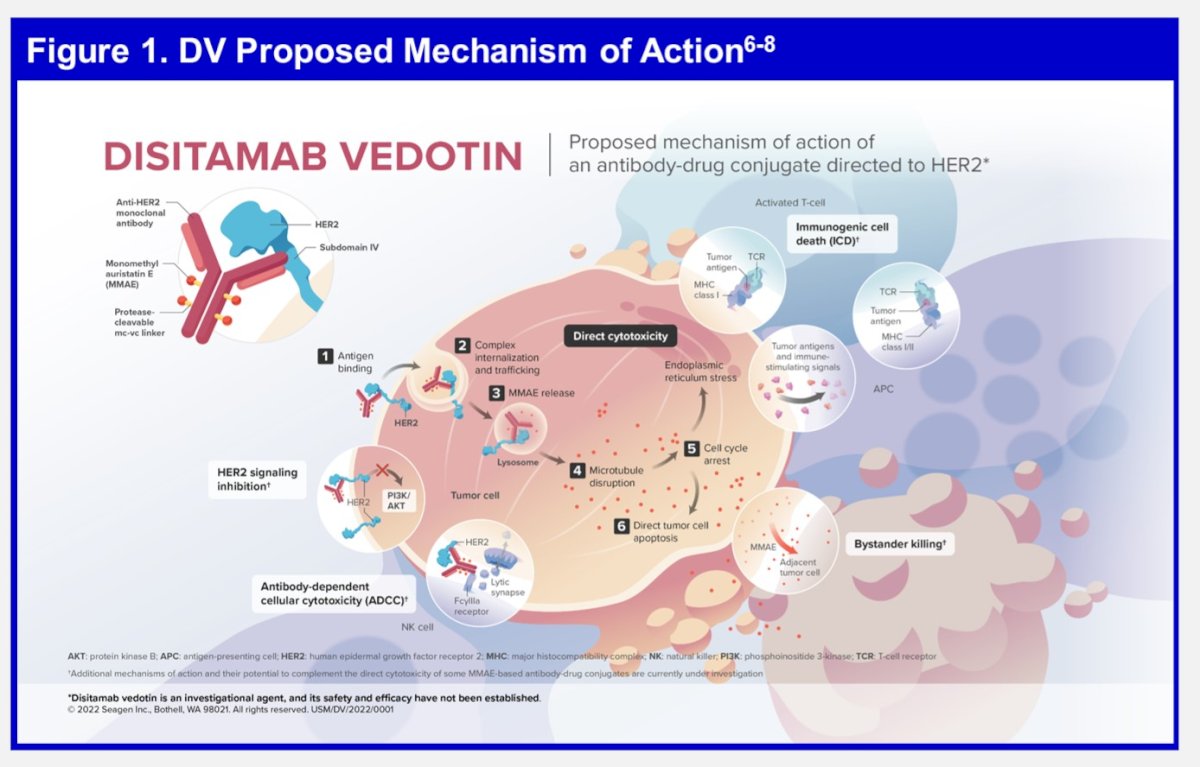
DV has shown encouraging activity with a manageable safety profile in a population of patients with HER2-expressing la/mUC:
- As a single agent in a post-platinum setting (IC 3+/2+; ORR, 50.5%; mPFS, 5.9 months; mOS, 14.2 months)2
- In combination with a PD-1 inhibitor in all comers (ORR, 76% in treatment-naive patients; 83.3%, 64.3%, and 33.3% in HER2 IHC 3+/2+, IHC 1+, and IHC O subgroups, respectively)3
DV is also being investigated in the post-platinum setting (NCT04879329). Dr. Galsky noted that these data overall provide a robust rationale for this phase 3 trial of DV plus pembrolizumab in the 1st line setting for HER2-expressing la/mUC.
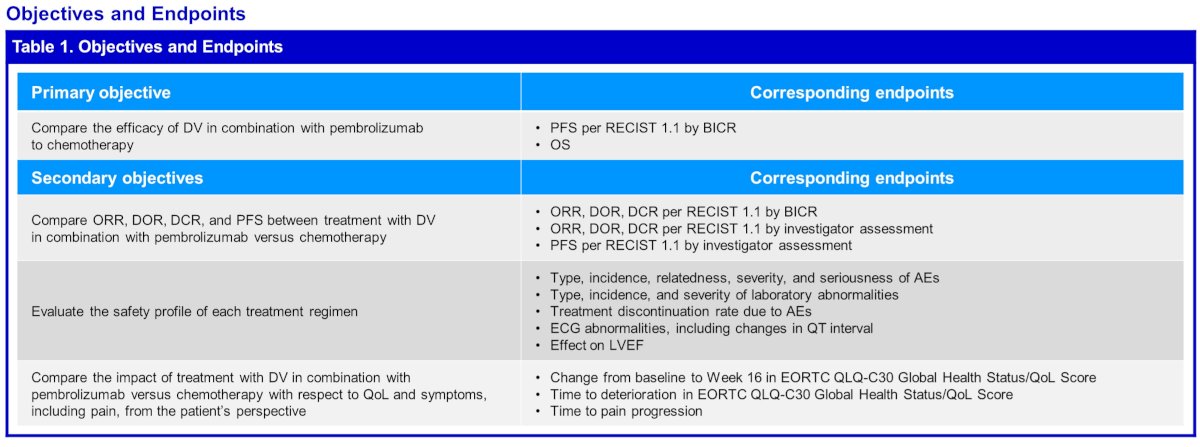
DV-001 (NCT05911295) is an open-label, randomized, multicenter, controlled phase 3 trial evaluating the combination of DV plus pembrolizumab versus chemotherapy in platinum-eligible patients with previously untreated HER2-expressing la/mUC.

The dual primary endpoints are progression-free survival (per RECIST via blinded independent central review) and overall survival. Secondary endpoints include:
- Objective response rate
- Duration of response
- Disease control rate
- Progression-free survival
- Safety
- Quality of life and symptoms
The full eligibility criteria are as follows:

The enrollment sites are illustrated below:

Presented by: Matt Galsky, MD, Professor of Medicine, Hematology and Medical Oncology, Director of Genitourinary Medical Oncology, Co-Director of the Center of Excellence for Bladder Cancer at The Tisch Cancer Institute, and Associate Director for Translational Research at The Tisch Cancer Institute, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References:
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024;390: 875-88.
- Sheng X, Wang L, He Z, et al. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J Clin Oncol. 2024;42(12): 1391-402.
- Sheng X, Zhou L, Yang K, et al. Disitamab vedotin, a novel humanized anti-HER2 antibody-drug conjugate (ADC), combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma: An open-label phase 1b/2 study. J Clin Oncol. 2023;41: Suppl 16.


