The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured an oral abstract session on bladder cancer, and a presentation by Dr. Jill Hamilton-Reeves discussing the primary results of S1600 assessing the effects of immune-enhancing nutrition on radical cystectomy outcomes. Radical cystectomy is associated with high complication rates, high infection rates, rapid muscle wasting after surgery, and peri-operative malnutrition.
The conceptual model for high postoperative complication rates is that a radical cystectomy induces a pro-inflammatory cytokine storm leading to decreased collagen, decreased nitric oxide, decreased T-cell function, and decreased protein translation:1-2
As such, SWOG S1600 was a randomized, double-blind, phase III trial comparing the impact of consuming specialized immunonutrition to oral nutritional support on postoperative complications following radical cystectomy. Specialized immunonutrition is fortified with nutrients, including L-arginine, omega-3 fatty acids, and dietary nucleotides.
Patients with bladder cancer planning to undergo radical cystectomy were enrolled and randomized 1:1 to specialized immunonutrition versus oral nutritional support, with the following clinical trial design: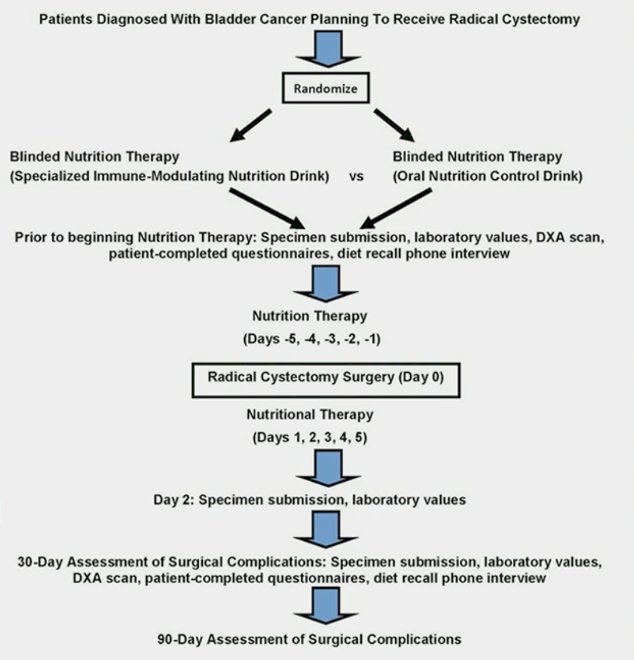
The primary endpoint was any (≥ grade I) postoperative complication assessed by the Clavien-Dindo scale at Day 30 after radical cystectomy. Multivariable logistic regression analysis was used, with the following stratification factors:
- Diversion type (neobladder versus conduit)
- Prior neoadjuvant chemotherapy (any vs none)
- Nutrition status (well nourished vs moderate malnutrition)
Additional outcomes included 90-day complications and high-grade (Clavien-Dindo ≥ Grade III) complications. Two-year progression-free survival and overall survival were assessed, with differences by arm reported using Log-rank test statistics. Protocol-specified intention-to-treat analyses based on all randomized patients and 1-sided alpha = 0.05 tests were performed.
Among 203 enrolled patients (99 on specialized immunonutrition, 104 on oral nutritional support), the median age was 68.8 years, 20% were female, and 5% were Black. Five patients did not meet eligibility criteria (1 on specialized immunonutrition, 4 on oral nutritional support) but were included under the intention to treat design: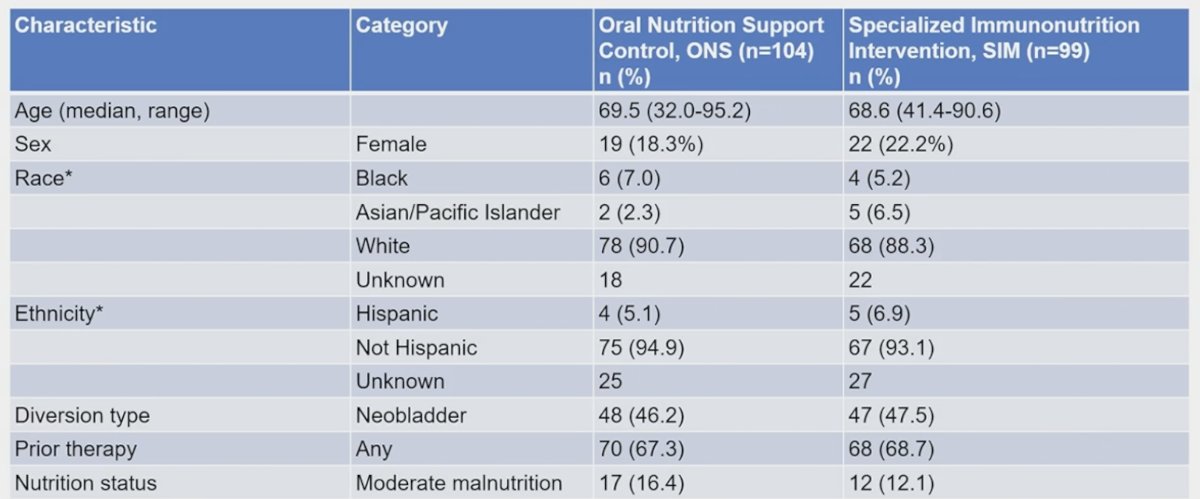
Seventeen patients withdrew consent prior to their Day 30 assessment, and 8 patients were not assessed. Thus, n = 178 (90 on specialized immunonutrition, 88 on oral nutritional support) were evaluable at Day 30 for the primary outcome. The proportion of patients experiencing Clavien-Dindo Grade ≥1 complications was 62.2% for specialized immunonutrition and 58.0% for oral nutritional support. In multivariable regression, there was no difference in Grade ≥1 complications by arm (OR 1.18, 95% CI, 0.64-2.18, 1-sided p = 0.71). There were no differences by arm in high-grade complications at Day 30, in any or high-grade complications at Day 90, or overall.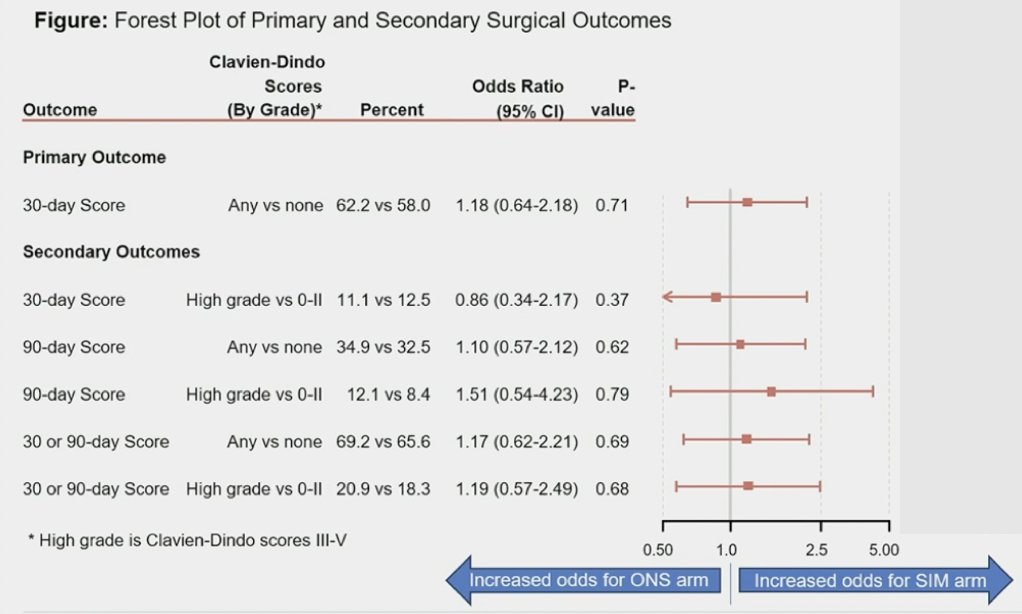
To date, among all 203 patients, 17 on specialized immunonutrition and 26 on oral nutritional support have progressed or died, with 2-year progression-free survival estimates of 77% and 68%, respectively (1-sided p = 0.16). Nine patients on specialized immunonutrition and 17 on oral nutritional support have died, with 2-year overall survival estimates of 87% and 78%, respectively (1-sided p = 0.07):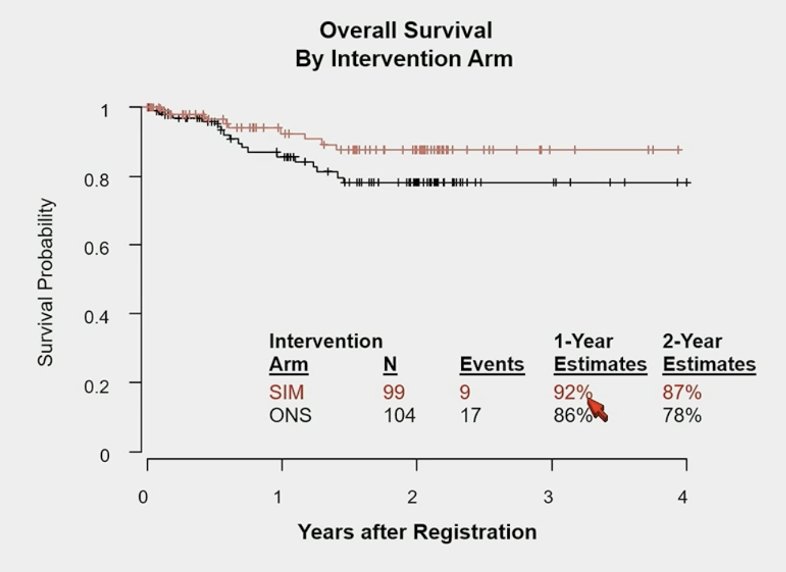
There were several limitations of this study: (i) Survival outcomes are both exploratory and not yet mature, so should be interpreted with caution (2 year protocol specified evaluation of survival outcomes with full follow-up is planned), (ii) focus on the overall rate of complications may dilute the more specific effects on infection rates or muscle mass, thus further analyses will be conducted, and (iii) nutrition biomarker data for adherence were collected but not yet evaluated.
Dr. Hamilton-Reeves concluded her presentation discussing the primary results of S1600 assessing the effects of immune-enhancing nutrition on radical cystectomy outcomes with the following take-home messages:
- There was no difference in any grade Clavien-Dindo complications by type of nutritional supplement for patients with bladder cancer undergoing radical cystectomy
- Fewer patients on the specialized immunonutrition arm have progressed or died, although differences were not statistically significant
- Follow-up for survival will continue through 3-years after enrollment
- Future work to understand the interaction of diet on cancers sensitive to immune modulation is needed
Presented by: Jill Hamilton-Reeves, PhD, RD, CSO, Associate Professor, University of Kansas Medical Center, Kansas City, MO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Hamilton-Reeves JM, Bechtel MD, Hand LK, et al. Effects of immunonutrition for cystectomy on immune response and infection rates: A pilot randomized controlled clinical trial. Eur Urol. 2016 Mar;69(3):389-392.
- Hamilton-Reeves JM, Stanley A, Bechtel MD, et al. Perioperative immunonutrition modulates inflammatory response after radical cystectomy: Results of a pilot randomized controlled clinical trial. J Urol. 2018 Aug;200(2):292-301.


