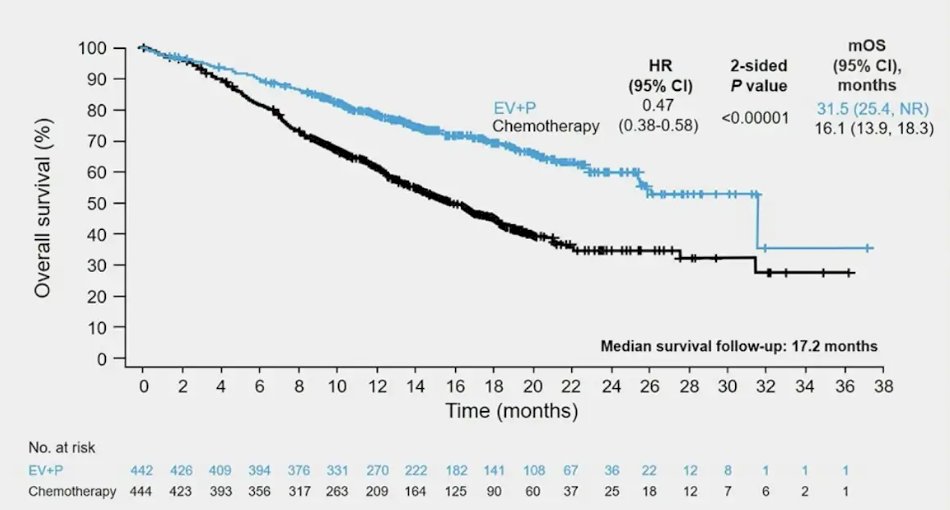
(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured an oral abstract session on urothelial carcinoma, and a presentation by Dr. Shilpa Gupta discussing patient reported outcomes from EV-302 assessing enfortumab vedotin + pembrolizumab versus platinum-based chemotherapy in previously untreated locally advanced or metastatic urothelial cancer. Patients with locally advanced or metastatic urothelial cancer have a poor prognosis with an estimated 5-year survival rate of <8% globally and have a high symptom and pain burden, which impacts their quality of life and functioning. Chemotherapy with gemcitabine + cisplatin or carboplatin has been the standard of care for first line treatment of locally advanced or metastatic urothelial cancer for decades. In 2023, enfortumab vedotin + pembrolizumab nearly doubled median progression-free survival and overall survival versus platinum-based chemotherapy in patients with previously untreated locally advanced or metastatic urothelial cancer in the phase 3 EV-302 trial:1
As such, enfortumab vedotin + pembrolizumab is an NCCN category 1, preferred treatment option in the first line treatment of previously untreated locally advanced or metastatic urothelial cancer. At ASCO 2024, Dr. Gupta and colleagues presented patient reported outcomes data.
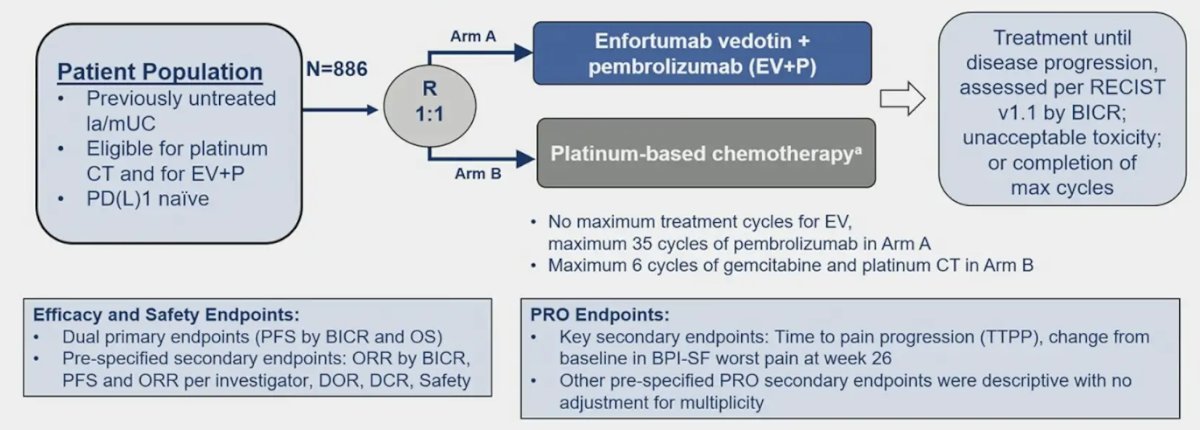
In EV-302 patients were randomized 1:1 to enfortumab vedotin plus pembrolizumab or platinum-based chemotherapy (gemcitabine with cisplatin or carboplatin). The trial design is highlighted below:
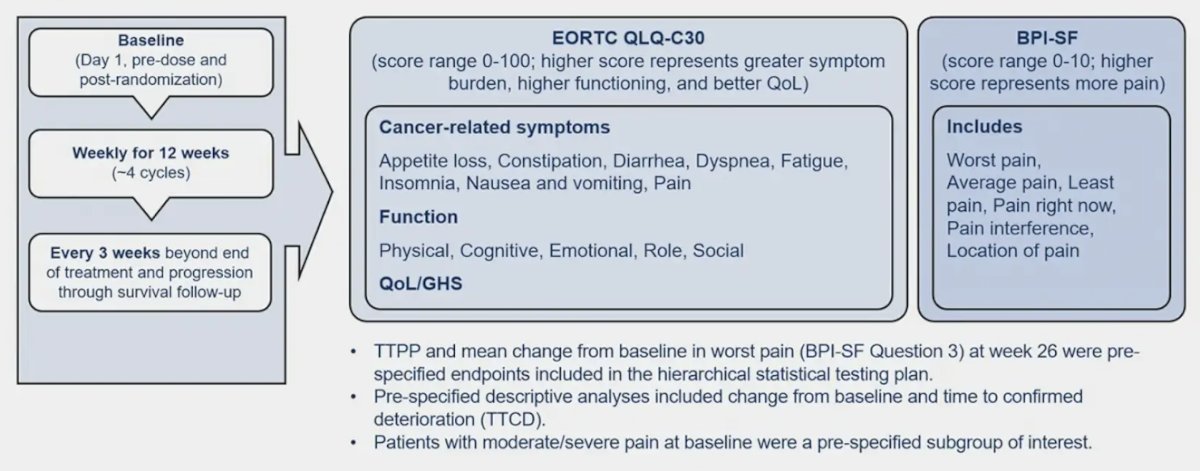
Patient reported outcome assessments included the EORTC Quality of Life Questionnaire (EORTC QLQ-C30), and the Brief Pain Inventory Short Form (BPI-SF) completed at baseline, weekly for 12 weeks, then every 3 weeks through survival follow-up, inclusive of the time post-progression:
Time to pain progression and mean change from baseline in worst pain at week 26 using the BPI-SF were prespecified analyses statistically tested using a gatekeeping strategy. Mean change from baseline through week 26 and time to confirmed deterioration of EORTC-QLQ-C30 and BPI-SF domains were prespecified descriptive analyses. Time to pain progression and time to confirmed deterioration were assessed using Kaplan-Meier methods.
Among the 886 patients randomized, 731 (376 received enfortumab vedotin plus pembrolizumab; 355 platinum-based chemotherapy) completed baseline patient reported outcome questionnaires. Baseline scores were balanced between treatment arms in the PRO full analysis set, and approximately 1/3 of patients had moderate to severe pain at baseline (pain score of 5 or great):

Compliance rates differed between arms and remained >70% through week 29 for enfortumab vedotin plus pembrolizumab and through only week 17 for platinum-based chemotherapy:

The median time to pain progression was previously presented in the primary manuscript,1 but to highlight was 14.2 months with enfortumab vedotin + pembrolizumab and 10.0 months with platinum-based chemotherapy (HR 0.92; 95% CI 0.72, 1.17; 2-sided p-value = 0.48). The least squares mean reduction in worst pain at week 26 was numerically greater with enfortumab vedotin + pembrolizumab versus platinum-based chemotherapy (-0.61 vs -0.03, least squares mean difference: -0.58 [95% CI -1.05, -0.11] [nominal 2-sided p-value = 0.015]).
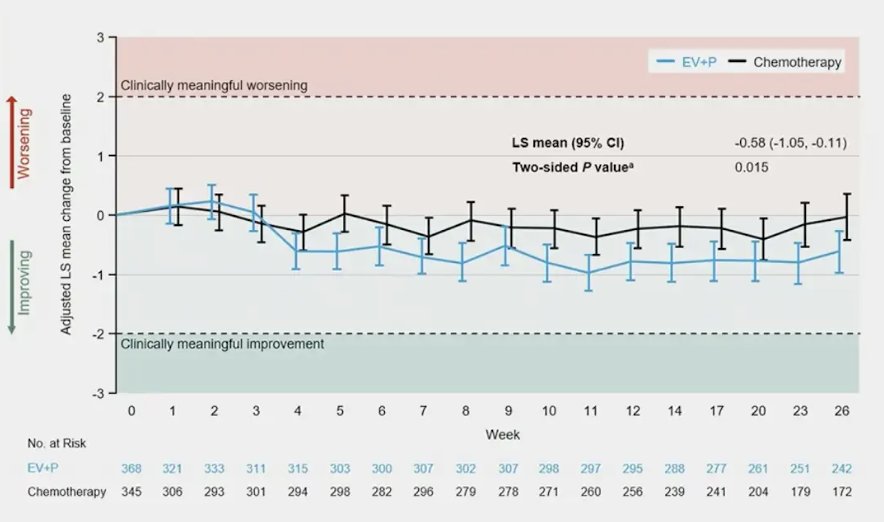
Patients with moderate to severe pain at baseline who were treated with enfortumab vedotin + pembrolizumab (n = 128, 34%) had a meaningful (>2 patient) improvement from baseline in BPI worst pain from week 3 through 26:
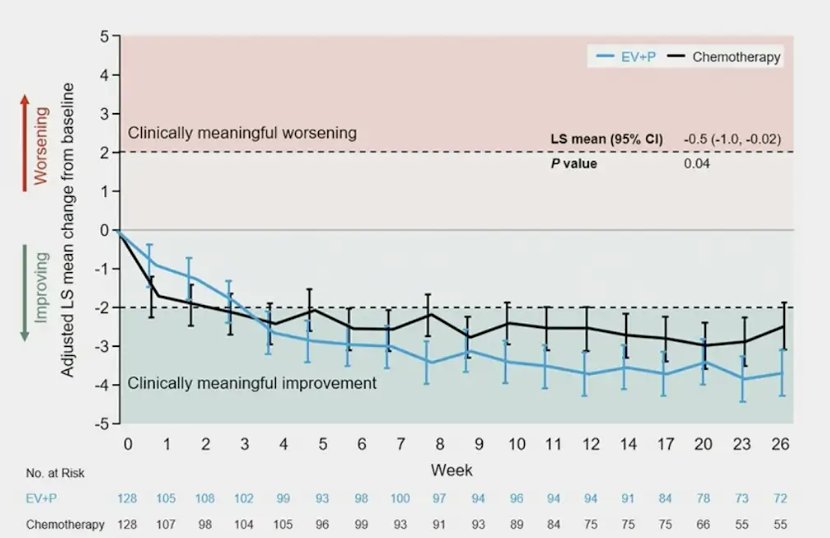
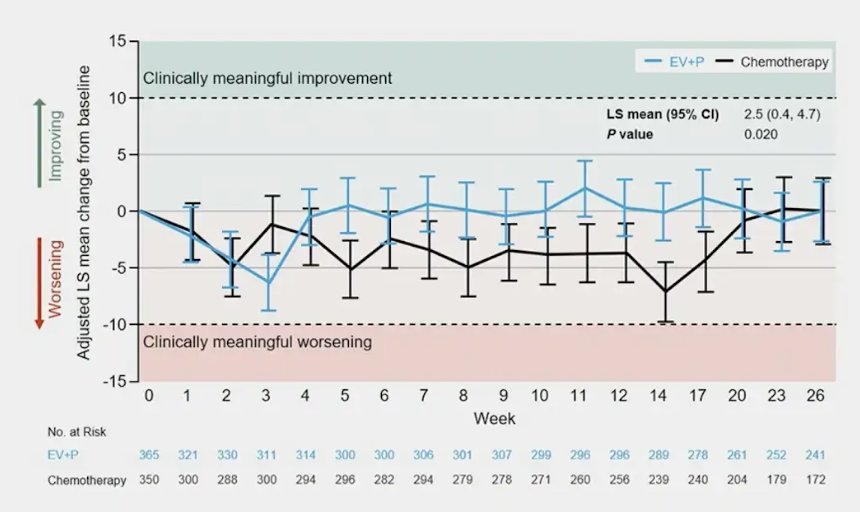
In EORTC QLQ-C30 Global Health Status/Quality of Life, enfortumab vedotin + pembrolizumab demonstrated a transient worsening at week 3 (-6.3) that returned to baseline from weeks 4 through 26, while patients treated with platinum-based chemotherapy demonstrated deterioration from week 1 through week 17 (range -1.2 to -7.1) when scores returned to baseline:
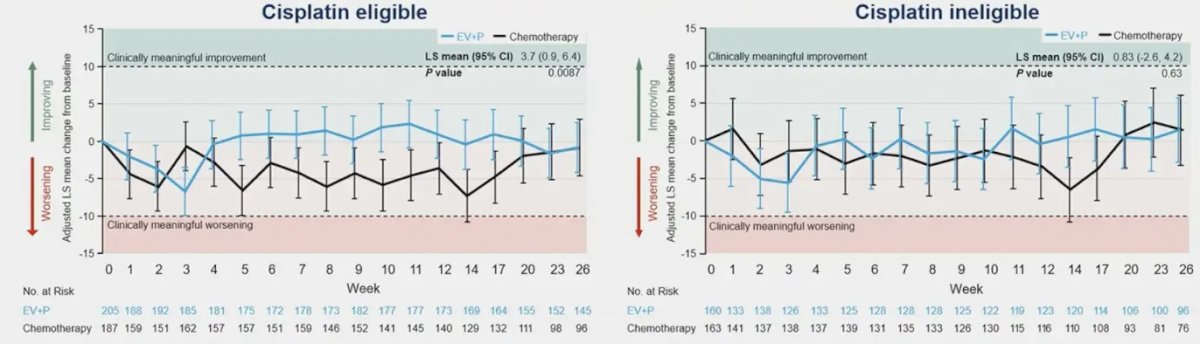
Both cisplatin eligible and cisplatin ineligible patients in the enfortumab vedotin + pembrolizumab arm demonstrated a transient worsening in Global Health Status/Quality of Life through week 3 that returned to baseline by week 4:
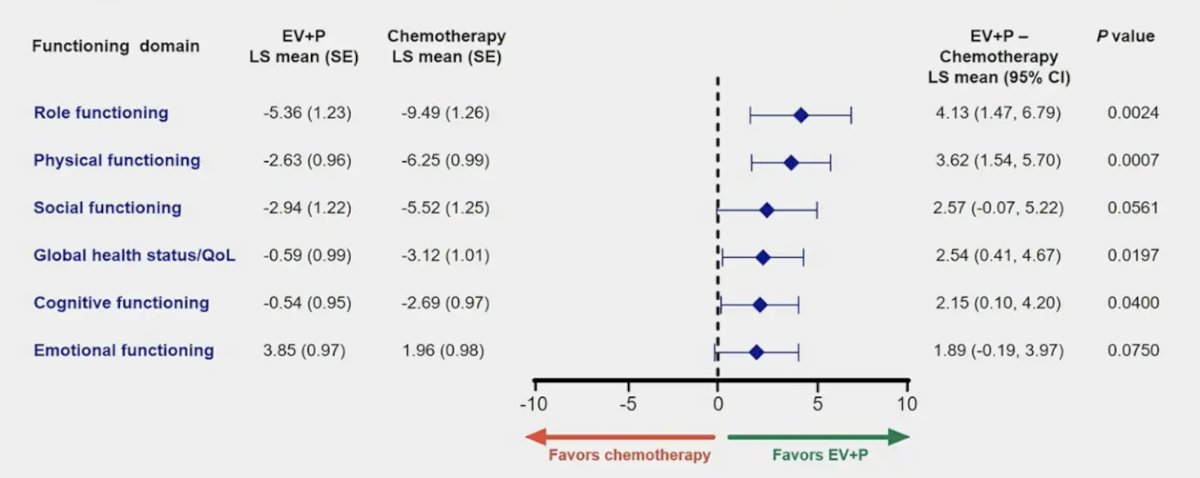
A summary of the change in EORTC QLQ-C30 functioning domains is highlighted below:
Time to confirmed deterioration for EORTC QLQ-C30 Global Health Status/Quality of Life was 5.9 months with enfortumab vedotin + pembrolizumab versus 3.2 months with platinum-based chemotherapy (HR 0.98, 95% CI 0.79 – 1.2).
Dr. Gupta concluded her presentation by discussing patient reported outcomes from EV-302 with the following take home messages:
- Patients treated with enfortumab vedotin + pembrolizumab have significantly improved progression free- and overall survival compared with platinum-based chemotherapy without detriment to quality of life and functioning
- Patients with moderate to severe pain treated with enfortumab vedotin + pembrolizumab demonstrated clinically meaningful improvements in worst pain and Global Health Status/Quality of Life
- Data collection across the entire patient journey was a notable approach and was associated with differences in compliance between treatment arms
- Findings from this study may inform the design of future trials
- Patient reported outcome data presented here complement the published clinical efficacy and safety data, add the patient perspective, and support the use of enfortumab vedotin + pembrolizumab for patients with previously untreated locally advanced or metastatic urothelial cancer
Presented by: Shilpa Gupta, MD, Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References: