(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on urothelial carcinoma, and a discussant presentation titled “Tailoring Treatment and Navigating Patient Preferences” by Dr. Samuel Takvorian discussing the following three abstracts: “Effects of immune-enhancing nutrition on radical cystectomy outcomes: Primary results from the randomized phase III double-blind clinical trial (S1600)” by Dr. Jill Hamilton-Reeves, “Patient-reported outcomes from a randomized, phase 3 trial of enfortumab vedotin plus pembrolizumab versus platinum-based chemotherapy in previously untreated locally advanced or metastatic urothelial cancer” by Dr. Shilpa Gupta, and “Impact of exposure on outcomes with enfortumab vedotin in patients with locally advanced or metastatic urothelial cancer” by Dr. Daniel Petrylak.
Dr. Takvorian notes one question a patient may have is: How can nutrition help me have the best possible outcome after surgery? He subsequently highlighted Dr. Hamilton-Reeves’ S1600 presentation discussing immune-enhancing nutrition. Unfortunately, this trial found that immune-enhancing nutrition does not impact post-operative outcomes based on a negative primary endpoint of no difference in any grade complications at 30 days. Moreover, there was no difference in secondary outcomes, including high-grade complications at 30 days and high/any grade complications at 90 days or 30/90 days respectively. Although immature, there was also no difference in progression-free survival and overall survival:
The strengths of this study include the robust research design including having a strong biologic rationale, double-blinded randomized clinical trial design, and having an active control. Additionally, there is biologic plausibility, however, Dr. Takvorian questions whether 10 days of perioperative nutrition was long enough. An additional limitation is that it is uncertain whether there was patient adherence. Dr. Takvorian also emphasized that there may be heightened relevance in the era of adjuvant immunotherapy, given that based on the CheckMate 274 trial, adjuvant immunotherapy is a new standard of care for high-risk muscle-invasive urothelial carcinoma after cystectomy.1 Furthermore, there is variability in efficacy of immunotherapy that may be explained by differences in the microbiome and dietary factors. This has already been somewhat shown in melanoma, where a Mediterranean-style diet is associated with higher immunotherapy response rate. Several future directions include:1 How can nutritional strategies improve perioperative performance and/or immunotherapy efficacy/safety, and Can this approach be personalized?2
Another question a patient may ask is “How am I going to feel on treatment?” To answer this, Dr. Takvorian discussed the patient-reported outcomes data from EV-302 presented by Dr. Shilpa Gupta. As we know, EV-302 showed paradigm-shifting benefits for enfortumab vedotin + pembrolizumab, specifically significant improvements in progression-free survival (HR 0.45, 95% CI 0.38-0.54) and overall survival (HR 0.47, 95% CI 0.38-0.58) compared to chemotherapy. However, there is differential treatment exposure in this trial: patients receiving enfortumab vedotin + pembrolizumab had a maximum of 35 cycles of pembrolizumab (with no maximum cycles of enfortumab vedotin), whereas patients receiving chemotherapy only had a maximum six cycles of chemotherapy. EV-302 showed that there was no difference in median time to pain progression across arms, which was the primary patient-reported outcome with pre-specified alpha allocation:
Dr. Takvorian notes that this is surprising given the marked progression-free survival advantage with enfortumab vedotin + pembrolizumab, likely owing to low rates of baseline pain (~1/3 moderate to severe pain). However, among patients with moderate to severe pain at baseline, there were meaningful improvements in pain and quality of life with enfortumab vedotin + pembrolizumab: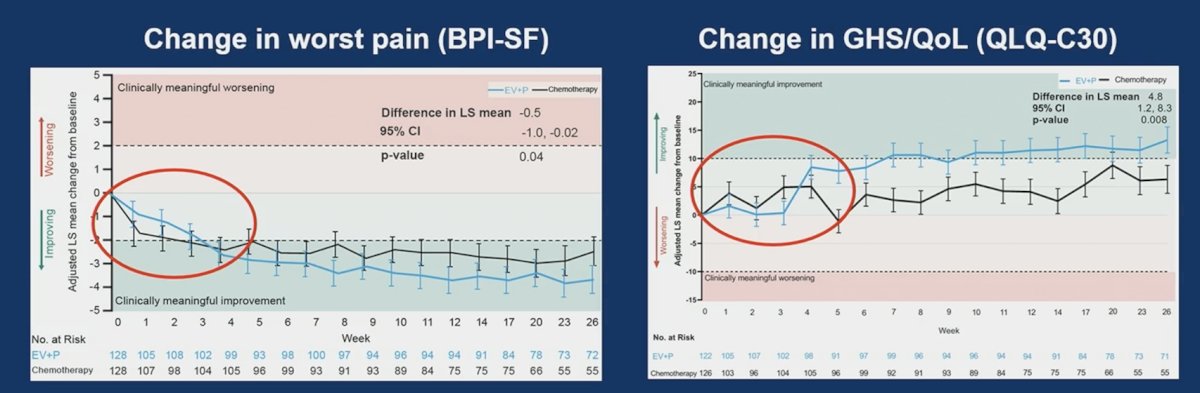
Dr. Takvorian notes that it is reassuring there is no meaningful worsening in Global Health Status/Quality of Life with enfortumab vedotin + pembrolizumab, but are we missing signals of treatment-related toxicity with a 2-item global quality of life measure?
- How would you rate your overall health during the past week?
- How would you rate your overall quality of life during the past week?
A single EORTC QLQ-C30 summary score may be more meaningful, reliable, and robust, but for now, we await the analysis of symptom domains (but key AESIs are missing such as rash and neuropathy). Do these data answer our patient’s question? Weighed against the unprecedented benefits of this regimen, EV-302 patient-reported outcome analysis supports enfortumab vedotin + pembrolizumab as a new standard of care for metastatic urothelial carcinoma. But, were these patient-reported outcome endpoints sufficient? Pain is a function of cancer control, and health-related quality of life is multidimensional, but as measured/reported it is unlikely to capture the burden of treatment-related toxicities of chronic therapy. A more comprehensive understanding of patient experience would also include:
- Individual patient-reported outcomes for relevant treatment-related adverse events (ie. NCI PRO-CTCAE)
- Overall measure of tolerability (ie. FACIT GP-5) – “I am bothered by side effects of treatment”
A third question a patient may ask us is “I’m worried about lowering the dose….will my treatment still work?” How can pharmacokinetic data address this patient’s concern? Dr. Petrylak and colleagues did an exploratory analysis of antibody-drug conjugate exposure on efficacy and safety outcomes across pivotal studies of enfortumab vedotin monotherapy. As a word of caution, Dr. Takvorian emphasized that these are post-hoc descriptive analyses and cross-trial comparisons. Medical oncologists are notorious for stating “dose reductions are necessary to improve tolerability, maintain drug delivery, and improve outcomes”, so do the pharmacokinetic data support this? Indeed, dose modifications were common in EV-201/EV-301: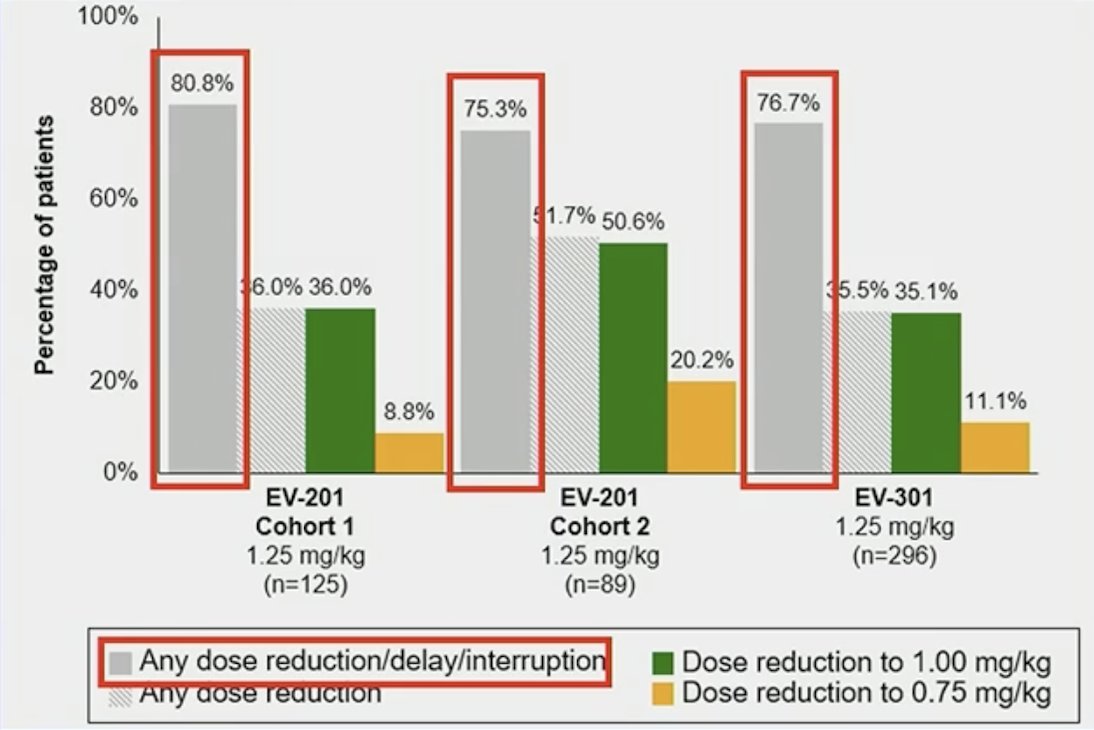
Encouragingly, this is in line with real-world enfortumab vedotin treatment patterns,2 whereby only 59% of patients received standard dose intensity (D1, 8, 15 of 28), with dose omissions most commonly at D15. In this real-world study, mean treatments per cycle were 2.4 (SD 0.5), and mean dose was 1.1 mg/kg/ (SD 0.2). In the analysis by Dr. Petrylak presented at ASCO 2024, across all exposure quartiles, enfortumab vedotin versus chemotherapy improved progression-free survival and overall survival in EV-301: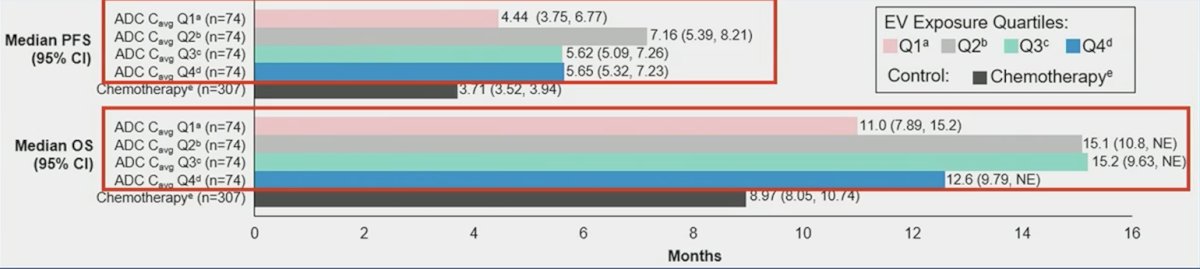
Furthermore, this was noted with similar durations of responses (albeit the caveat of small numbers in these groups). Dr. Takvorian stated that future directions may include optimal schedule and de-escalation strategies among responders: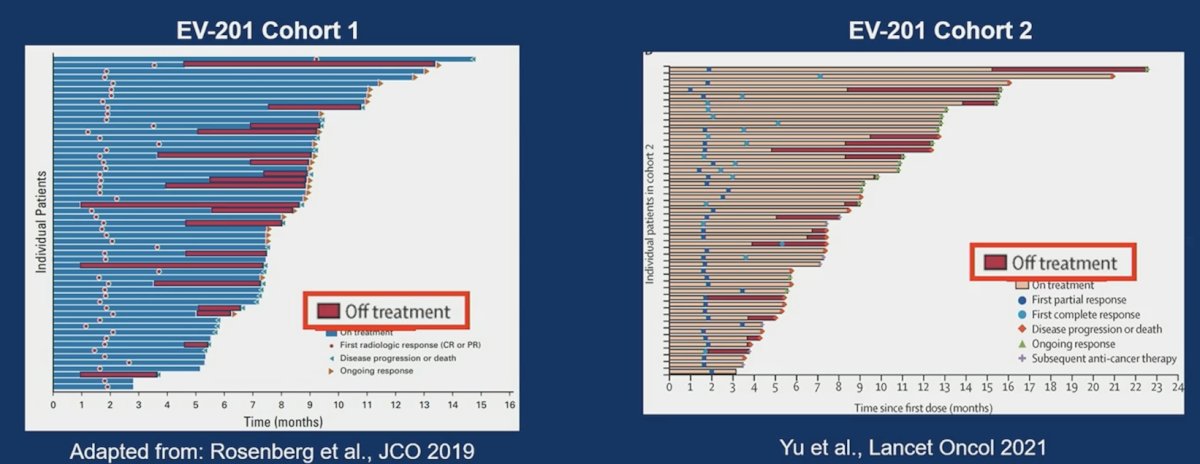
Dr. Pooja Ghatalia is the PI on the NCT05923190 trial “A Pilot Trial of Enfortumab Vedotin Schedule De-escalation in Metastatic Urothelial Carcinoma” looking at reducing treatment burden among patients responding to enfortumab vedotin + pembrolizumab over time: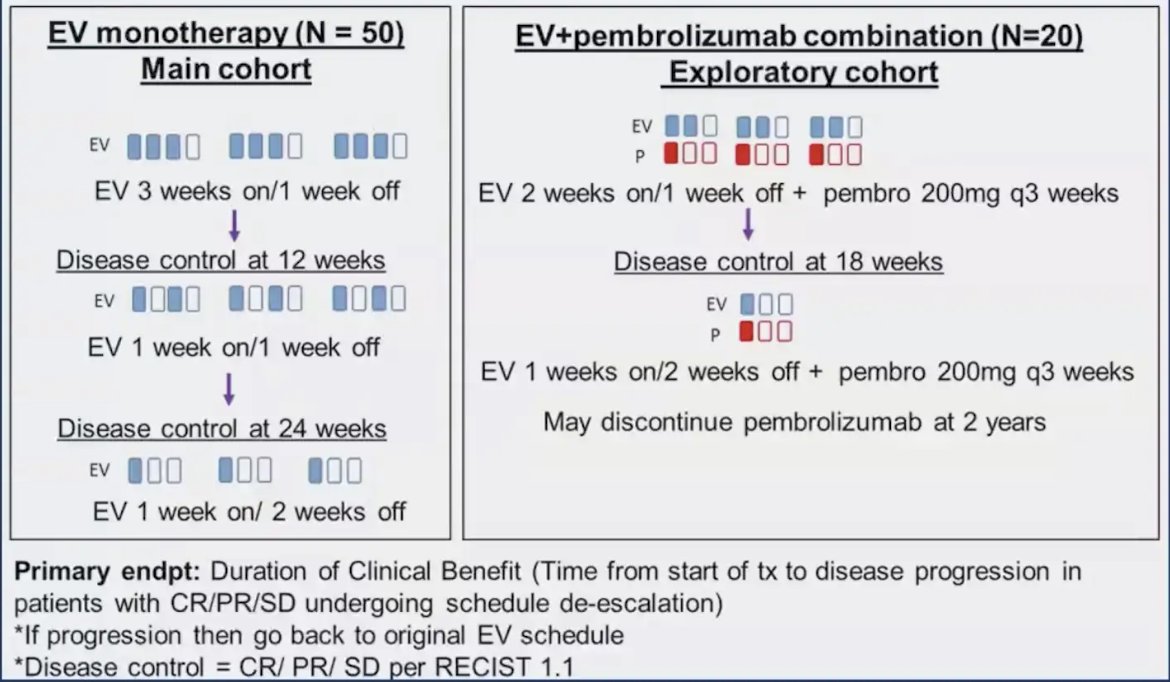
Dr. Takvorian concluded his presentation with the following take-home messages:
- For the patient question: How can nutrition help me have the best possible outcome after surgery? Our answer at this time may be “For now, the best we can advise is a heart-healthy diet with protein-calorie supplementation – but studies are ongoing to see how nutrition might optimize your surgical recovery, immune response, and long-term outcomes”
- For the patient question: How am I going to feel on treatment? Our answer at this time may be “While there are important side effects that require monitoring and management, overall patients tolerate enfortumab vedotin + pembrolizumab without major detriment to their quality of life or functioning. Often patients will have meaningful improvements in pain”
- For the patient question: I’m worried about lowering the dose….will my treatment still work? Our answer at this time may be “Altering the dose or schedule of your treatment is an important way for us to manage side effects. Dose modifications were used frequently in the studies leading to enfortumab vedotin’s approval, and the drug maintains activity even at lower dose levels”
Presented by: Samuel U. Takvorian, MD, MS, Assistant Professor of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
- Mamtani R, Tsingas K, Parikh RB, et al. Real-world use, dose intensity, and adherence to enfortumab vedotin in locally advanced or metastatic urothelial cancer. Urol Oncol. 2024 Jun;42(6):177.e1-177.e.4.


