(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on bladder cancer, and a presentation by Dr. Yohann Loriot discussing long-term outcomes of avelumab from the JAVELIN Bladder 100 trial in patients with histological subtypes. Results from the phase 3 JAVELIN Bladder 100 trial led international treatment guidelines to recommend avelumab first-line maintenance as a standard-of-care treatment for patients with advanced urothelial carcinoma without progression after first-line platinum-based chemotherapy.1
Around 20% of urothelial carcinomas are mixed with other histological subtypes/variants, and these tumors do not have specific treatment guidelines and represent an unmet treatment need. In the large real-world AVENANCE study (NCT04822350; n = 594) of avelumab first-line maintenance in patients with advanced urothelial carcinoma in France, outcomes in patients with histological subtypes were consistent with those in the overall population [ESMO 2023]. At the 2024 ASCO annual meeting, Dr. Loriot and colleagues reported a post hoc analysis of long-term outcomes from JAVELIN Bladder 100 in patients with predominantly urothelial carcinoma mixed with <50% histological subtype component.
The JAVELIN Bladder 100 trial enrolled patients that had unresectable locally advanced or metastatic urothelial carcinoma without progression after first-line platinum-based chemotherapy and were randomized 1:1 to receive avelumab + best supportive care or best supportive care alone: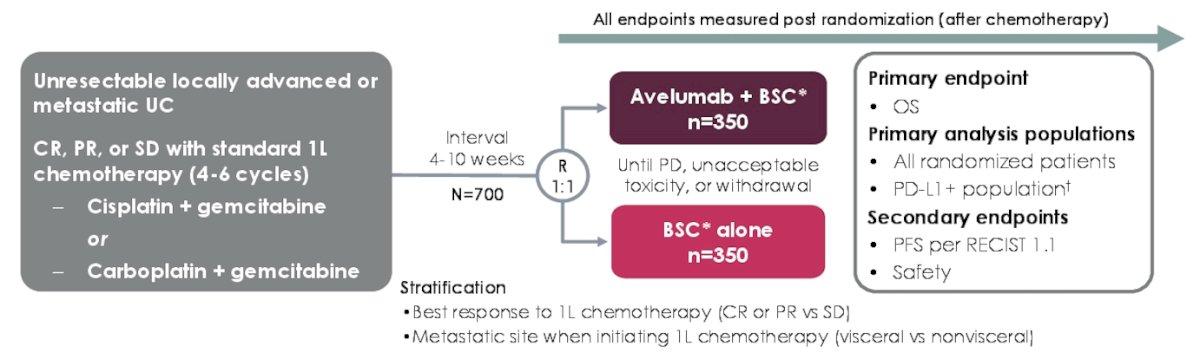
The primary endpoint was overall survival measured from randomization, whereas secondary endpoints included progression-free survival and safety. This post hoc analysis was conducted in all patients with histological subtypes. Aggressive histologic variants, such as plasmacytoid and sarcomatoid, or divergent differentiation (ie. squamous, glandular, and neuroendocrine) were not excluded from this analysis.
In the avelumab + best supportive care and best supportive care alone arms, respectively, 44/350 and 57/350 patients had histological subtypes. At efficacy data cutoff (June 4, 2021), median follow-up in both arms was ≥ 38.0 months. Baseline characteristics of patients with histologic subtypes and the overall trial population are shown in the following table: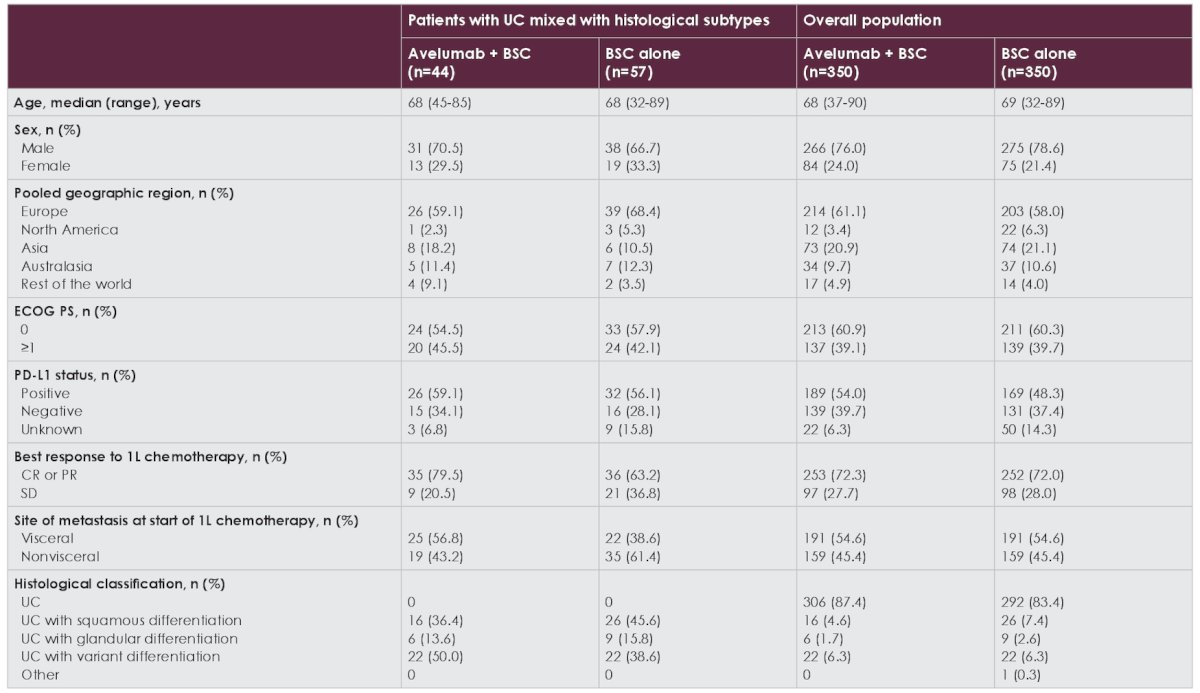
Among patients with histologic subtypes, a slightly higher proportion in the avelumab + best supportive care arm versus the best supportive care alone arm had visceral metastases (56.8% vs 38.6%) and a complete or partial response to first-line chemotherapy (79.5% vs 63.2%). In patients with histological subtypes, overall survival was prolonged in the avelumab + best supportive care versus best supportive care alone arm (HR 0.74, 95% CI 0.44-1.24):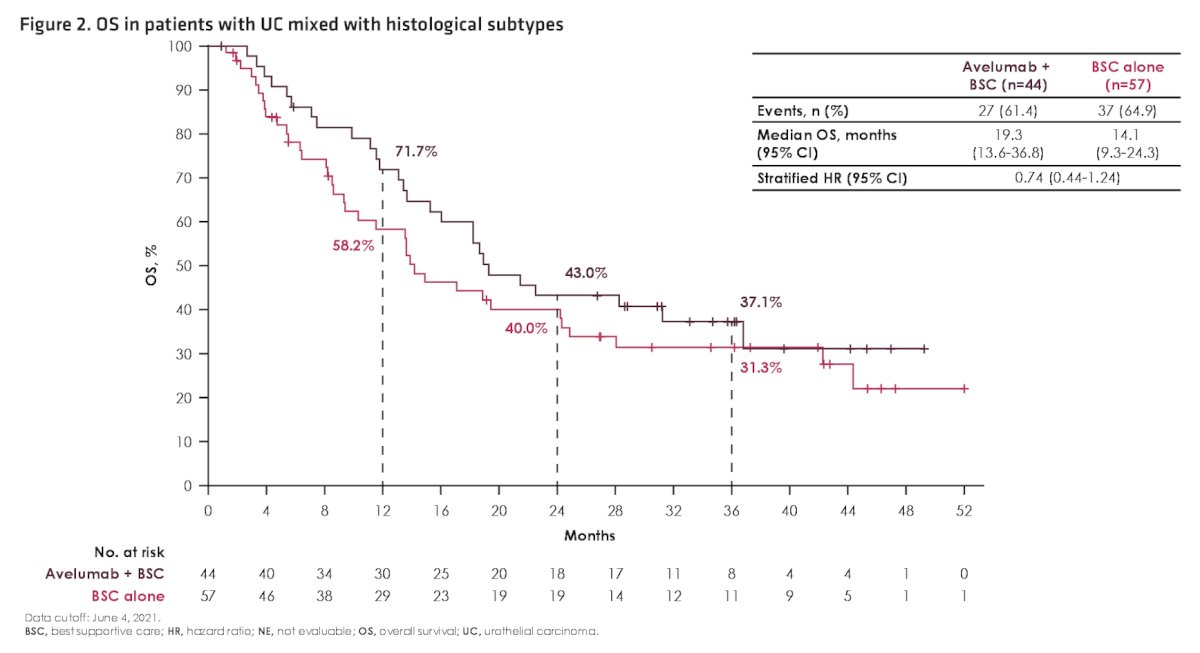
Investigator assessed progression-free survival was also prolonged with avelumab + best supportive care versus best supportive care alone in patients with histologic subtypes (HR 0.52, 95% CI 0.33-0.83):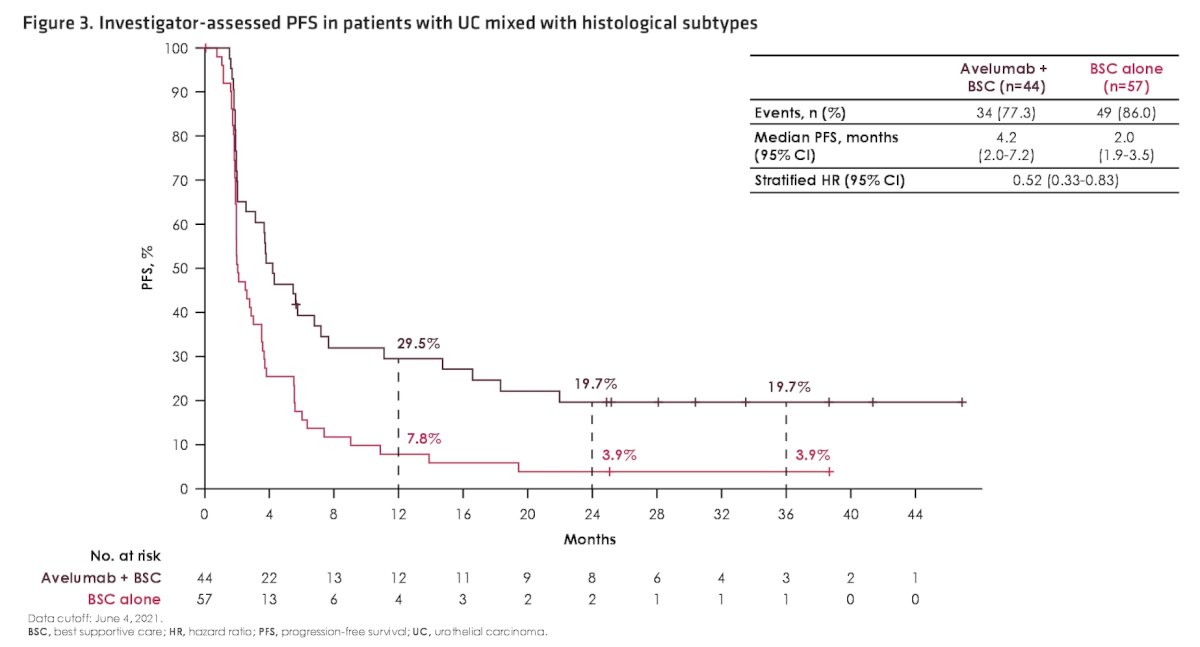
Long-term safety (cutoff, April 6, 2023) in treated patients with histological subtypes was generally consistent with the overall safety population. Treatment-related adverse events of any grade occurred in 36 patients (83.7%) in the avelumab + best supportive care arm vs 1 patient (1.8%) in the best supportive care alone arm. Grade ≥3 treatment-related adverse events occurred in 9 patients (20.9%) and 0 patients, respectively: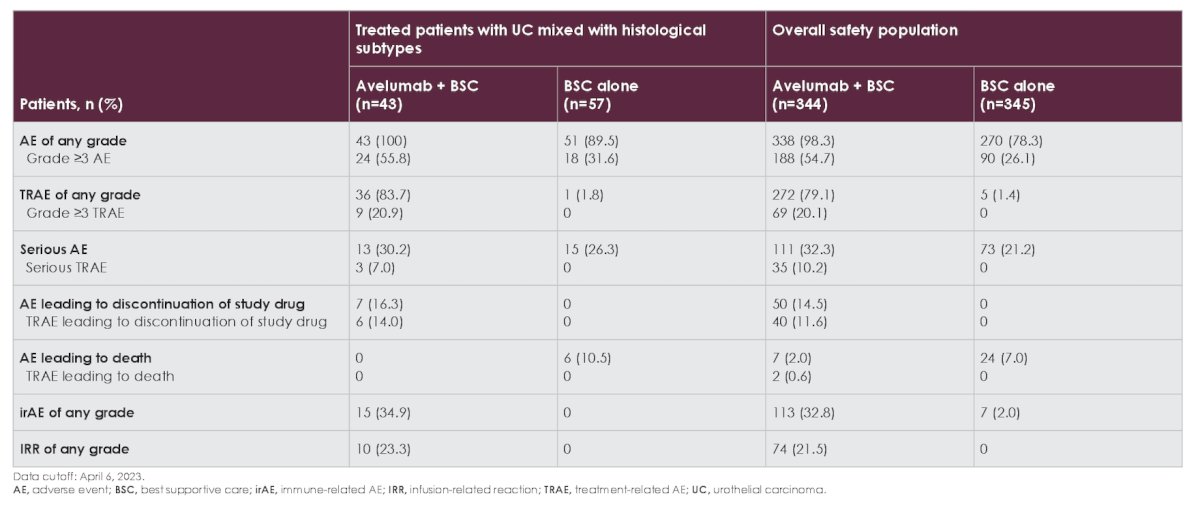
Dr. Loriot concluded his presentation discussing long-term outcomes of avelumab from the JAVELIN Bladder 100 trial in patients with histological subtypes with the following take-home messages:
- This exploratory analysis shows the long-term efficacy and safety of avelumab first-line maintenance in patients with histological subtypes in JAVELIN Bladder 100
- No new safety concerns were identified
- These results were consistent with those in the overall population and support the use of avelumab first-line maintenance in patients with advanced urothelial carcinoma without progression following first-line platinum-based chemotherapy, including patients with predominantly urothelial carcinoma mixed with <50% histological subtype component
Presented by: Yohann Loriot, MD, PhD. Physician Scientist; Director of Bladder Cancer Program, Gustave Roussy, Université Paris-Saclay, Villieuf, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Reference:
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.


