(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on bladder cancer, and a presentation by Dr. Syed Arsalan Ahmed Naqvi discussing cost-effectiveness analysis of contemporary first-line agents in locally advanced/metastatic urothelial carcinoma. EV-3021 and CheckMate 9012 demonstrated significant survival benefit with enfortumab vedotin + pembrolizumab and gemcitabine + cisplatin + nivolumab as first-line therapy, respectively, in patients with locally advanced/metastatic urothelial carcinoma. However, whether these treatments are cost-effective or not remains unclear.
A three-state Markov model (progression-free, progression, and death) was developed, and state utilities were derived from published literature. State transition probabilities were informed from point probabilities and hazard ratios for overall survival and progression-free survival obtained from the latest follow-up of eligible trials. Average sales price (2024 USD) for individual treatments (excluding ancillary charges) were obtained from the center of Medicare and Medicaid Services (US payer’s perspective):
Treatment strategies were modeled in accordance with the dose/schedule reported in the eligible trials. Patients who received gemcitabine + cisplatin or gemcitabine + carboplatin and did not progress were modeled to receive avelumab maintenance. Half-cycle corrected costs and utilities were accrued over a 10-year lifetime horizon and were discounted at 3%. Monte Carlo simulation was used to estimate quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios. A willingness-to-pay threshold of $100,000 per QALY was used.
In cisplatin-eligible patients, gemcitabine + cisplatin + nivolumab demonstrated a 0.8 QALY gain over gemcitabine + cisplatin at an incremental cost-effectiveness ratio of 21,127 ($/QALY). Gemcitabine + cisplatin + nivolumab was also associated with an incremental net monetary benefit of $63,099 relative to gemcitabine + cisplatin. Enfortumab vedotin + pembrolizumab demonstrated a 2.4 QALY gain over gemcitabine + cisplatin at an incremental cost-effectiveness ratio of 915,283 and a 1.6 QALY gain over gemcitabine + cisplatin + nivolumab at an incremental cost-effectiveness ratio of 1,406,308. In cisplatin-ineligible patients, enfortumab vedotin + pembrolizumab demonstrated a 2.4 QALY gain over gemcitabine + carboplatin at an incremental cost-effectiveness ratio of 934,761.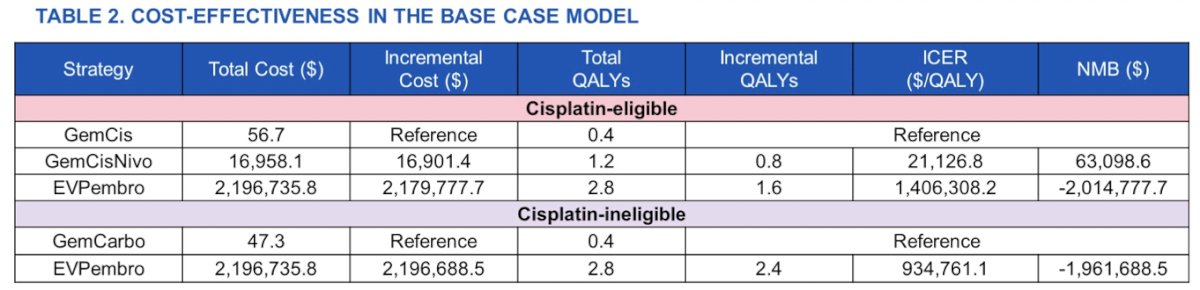
Sensitivity analysis using a total of 12 cycles for enfortumab vedotin + pembrolizumab showed cost-effectiveness at an incremental cost-effectiveness ratio of 69,594 relative to gemcitabine + cisplatin + nivolumab in cisplatin-eligible patients, and at an incremental cost-effectiveness ratio of 53,099 relative to gemcitabine + carboplatin in cisplatin-ineligible patients: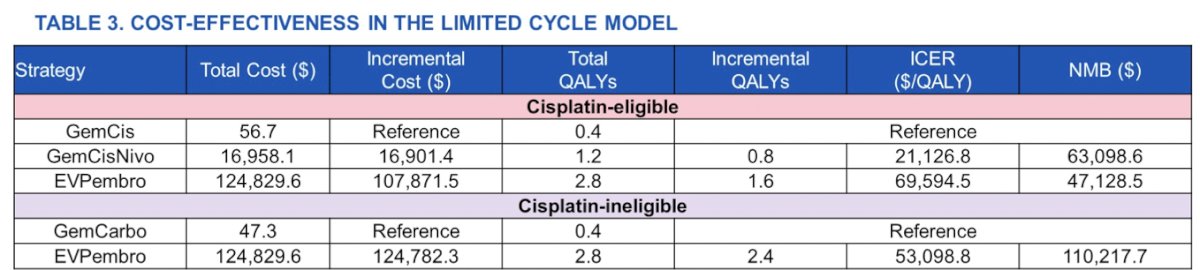
Price threshold analyses demonstrated that enfortumab vedotin + pembrolizumab is likely to be cost-effective at a cost of $12,344 for cisplatin-eligible and $13,374 per cycle for cisplatin-ineligible patients (original cost: $35,665.60 per cycle). The cost-effectiveness acceptability curves are as follows: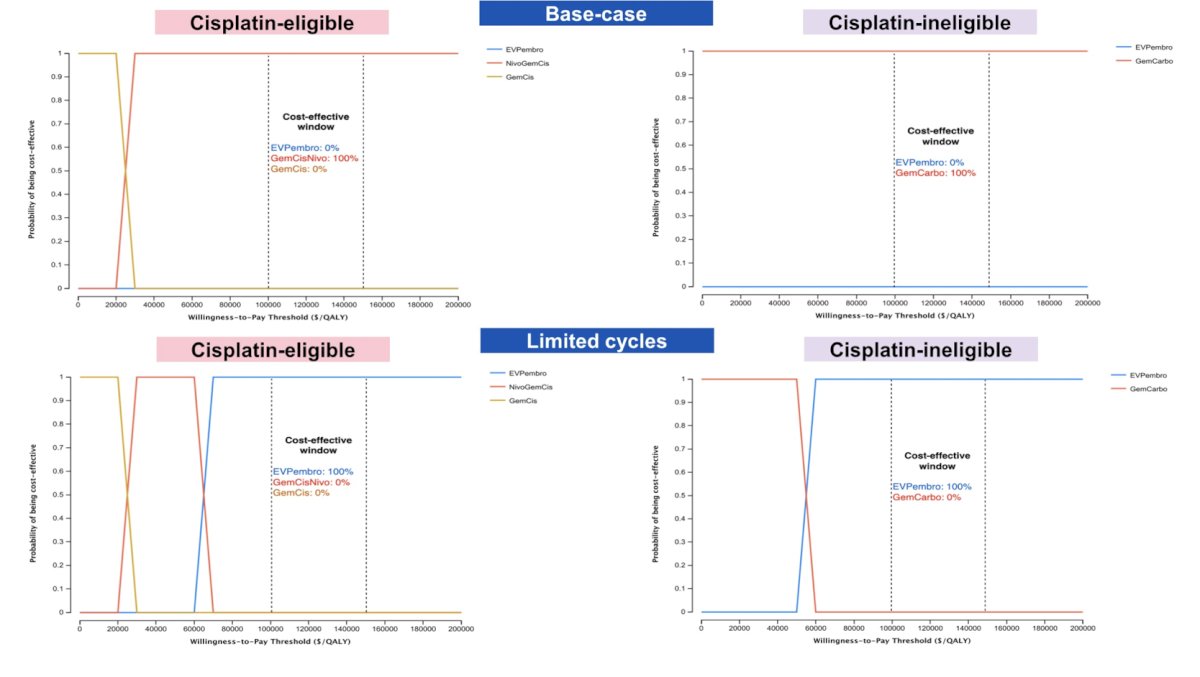
Dr. Naqvi concluded his presentation discussing the cost-effectiveness analysis of contemporary first-line agents in locally advanced/metastatic urothelial carcinoma with the following take-home messages:
- In terms of US payer’s perspective, gemcitabine + cisplatin + nivolumab is most likely to be cost-effective in cisplatin-eligible patients at the willingness-to-pay threshold of $100,000/QALY
- Limiting the number of cycles for enfortumab vedotin + pembrolizumab may render it as a cost-effective first-line treatment in locally advanced/metastatic urothelial carcinoma patients regardless of cisplatin-eligibility status
Presenter: Syed Arsalan Ahmed Naqvi, Fellow, Division of Hematology and Oncology, Department of Internal Medicine, Mayo Clinic, Phoenix, AZ
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10)875-888.
- Van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789.


