(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on bladder cancer, and a presentation by Dr. Arlene Siefker-Radtke discussing FGFR3 alterations in patients who develop locally advanced or metastatic urothelial cancer, and their association with tumor subtype and clinical outcomes in patients treated with erdafitinib versus pembrolizumab.
Erdafitinib, an oral pan-FGFR tyrosine kinase inhibitor is approved to treat adult patients with locally advanced or metastatic urothelial cancer with susceptible FGFR3 alterations, whose disease progressed on or after at least one line of prior systemic therapy. In superficial and surgically resectable urothelial carcinoma, FGFR3 alteration has been shown to be highly associated with luminal subtype characterized by expression of luminal markers, low expression of basal markers, and with immune cold/poor immune infiltration. In a randomized open-label phase III THOR study (Cohort 2), erdafitinib showed improvement in objective response and progression-free survival compared to pembrolizumab, with no significant improvement in overall survival.1 Exploration of subtypes in a large cohort of metastatic urothelial cancer patients has not been done and so a molecular analysis was undertaken (Cohort 2) to further understand the clinical findings.
All available tumors from patients enrolled in THOR Cohort 2 (FGFR3 alteration positive; n = 201) and a subset of FGFR wildtype (n = 116), who were anti-PD-L/PD1 naïve, were used to perform whole transcriptome RNA sequencing, where 152 and 84, respectively, passed quality control: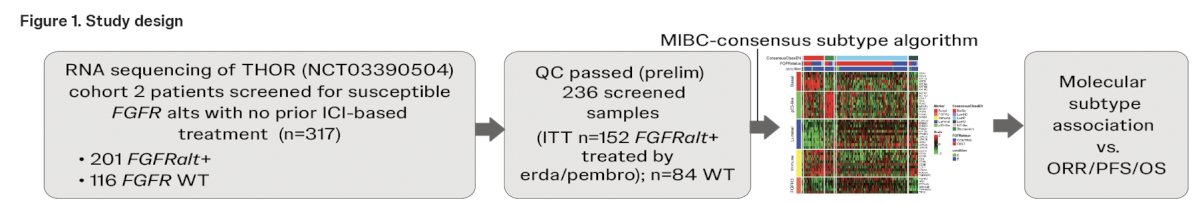 FGFR status was determined using the Qiagen therascreen FGFR RT-PCR test. The consensus single-sample classifier was applied to the RNAseq data to determine molecular subtypes. Tumor subtypes were correlated with treatment response to erdafitinib or pembrolizumab, progression-free survival, and overall survival.
FGFR status was determined using the Qiagen therascreen FGFR RT-PCR test. The consensus single-sample classifier was applied to the RNAseq data to determine molecular subtypes. Tumor subtypes were correlated with treatment response to erdafitinib or pembrolizumab, progression-free survival, and overall survival.
In erdafitinib- and pembrolizumab-treated groups, the majority of patients harbored an FGFR mutation (76.9% and 80.5%) versus translocation (23.1% and 17.2%), respectively: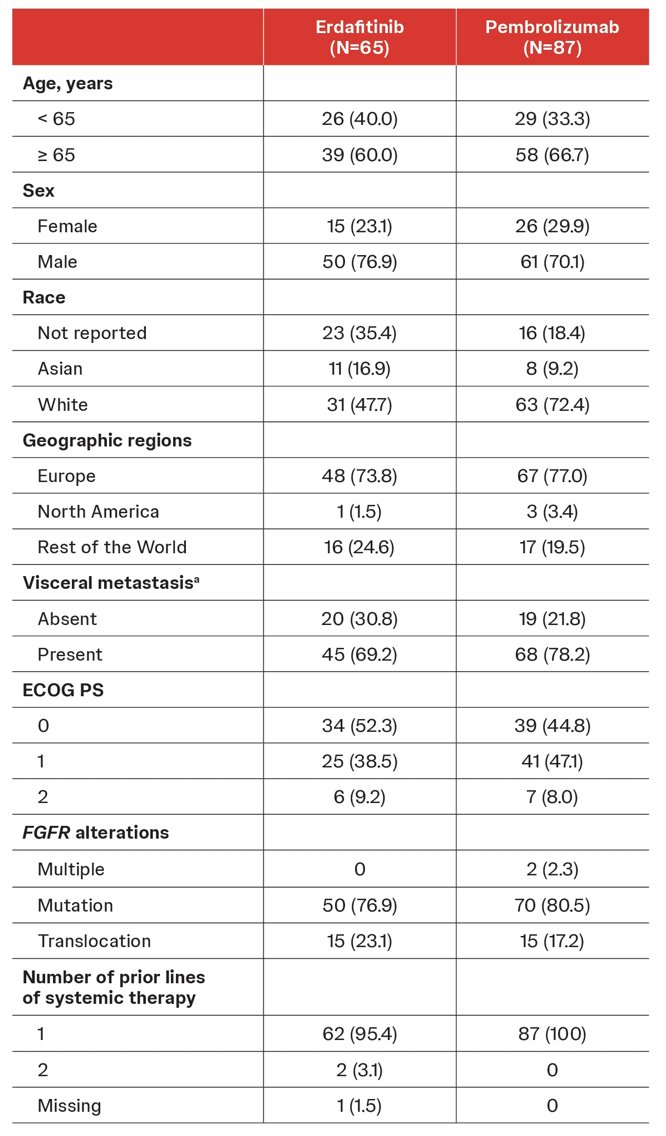
Molecular classification of tumors identified a significant proportion of luminal-papillary subtype in the tumors harboring FGFR3 alteration compared to FGFR wild-type (78.3% vs. 36.9%, p < 0.001) versus other subtypes: basal/squamous (11.2% vs. 31.0%), stroma-rich (6.6% vs. 10.7%), neuroendocrine-like (0% vs. 2.4%), luminal-unstable (3.3% vs. 17.9%) and luminal-unspecified (0.7% vs. 1.2%), respectively: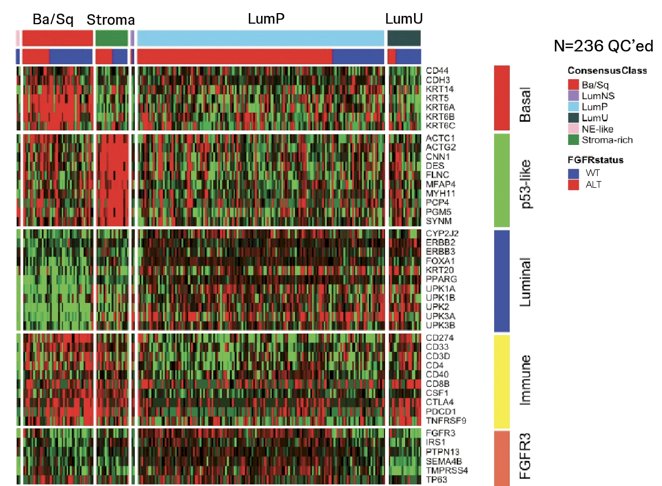

Consistently, FGFR3 alteration type showed differential association with subtypes: 3.2% of fusions and 14.1% of mutations were detected in basal/squamous subtype while 74.2% and 79.3%, respectively, were detected in luminal-papillary: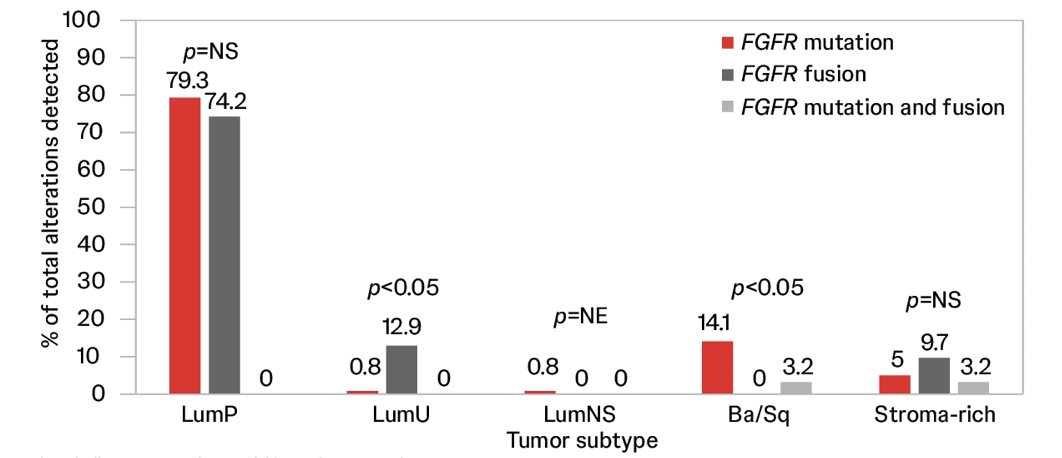
Clinical outcomes evaluated within luminal-papillary subset showed a significant improvement in objective response rate of erdafitinib-treated versus pembrolizumab-treated patients (41.7 vs. 19.7%; p = 0.01), which was consistent with the intent to treat population (40.0% vs. 21.6%):
Numerical improvement was observed in progression-free survival in the luminal-papillary subtype between erdafitinib versus pembrolizumab (5.5 vs 2.7 months) compared with the intent to treat population (4.4 vs 2.7 months):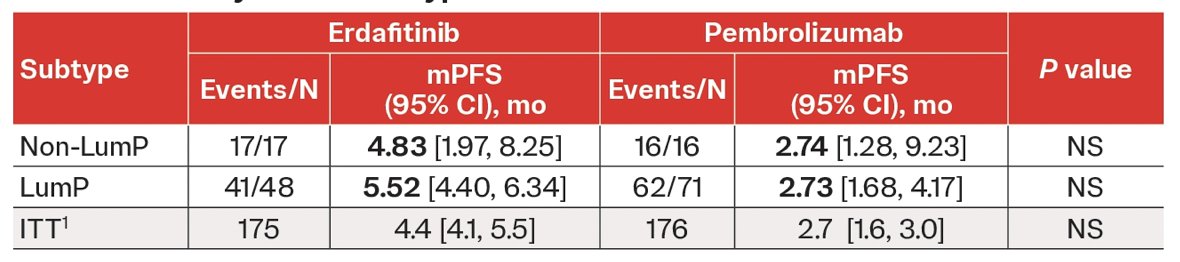
However, the benefit of erdafitinib over pembrolizumab in the luminal-papillary subtype did not translate to overall survival (10.9 vs. 12.9 months), similar to the intent-to-treat population (10.9 vs. 11.1 months):
Dr. Siefker-Radtke concluded her presentation discussing FGFR3 alterations in patients who develop locally advanced or metastatic urothelial cancer, and their association with tumor subtype and clinical outcomes in patients treated with erdafitinib versus pembrolizumab with the following take-home messages:
- Molecular subtypes were evenly distributed in FGFR wild-type tumors with a large proportion constituting the luminal-papillary subtype
- Luminal-papillary subtype was enriched in FGFR-altered tumors
- Both FGFR mutations and fusions enrich for the luminal-papillary tumor subtype
Presented by: Arlene O. Siefker-Radtke, MD, Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Reference:
- Siefker-Radtke AO, Matsubara N, Park SH, et al. Erdafitinib versus pembrolizumab in pretreated patients with advanced or metastatic urothelial cancer with select FGFR alterations: Cohort 2 of the randomized phase III THOR trial. Ann Oncol. 2024 Jan;35(1):107-117


