(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Session: Managing Variant Histologies in Urothelial and Renal Cell Cancers. Dr. Jean Hoffman-Censits delve into what is considered variant Histology urothelial cancer and what are the available treatment options in 2024.
The landscape of first-line management for urothelial cancer has radically changed over the past year and continues to evolve. With the approval of new agents like enfortumab vedotin (EV) combined with pembrolizumab, as well as new regimens such as nivolumab and gemcitabine cisplatin (GC), there are now a variety of options for treating patients with urothelial cancer (UC).(1,2) However, the majority of trials leading to these changes and updates in clinical practice have predominantly included patients with pure UC. A major limitation of these trials is that they often lump variant histology (VH) into a single category rather than splitting outcomes data into different variants. This approach assumes that "variant histology" is a single entity, ignoring the fact that many variants exist, each with distinct prognoses, outcomes, and responses to platinum-based chemotherapy or immunotherapy.
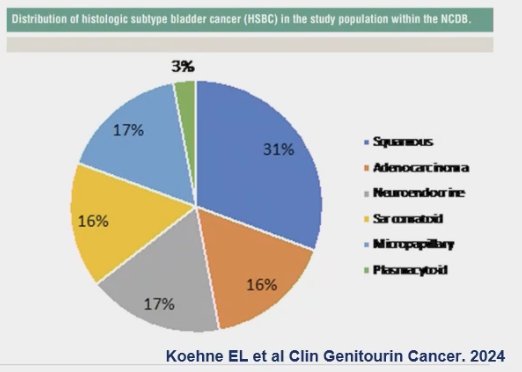
The most common VH is squamous cell carcinoma, which was found to represent 31% of VH in a recent report on the distribution of histologic subtypes of bladder cancer. This was followed by neuroendocrine (17%), adenocarcinoma and sarcomatoid variants (16%), with the least common being plasmacytoid.(3)
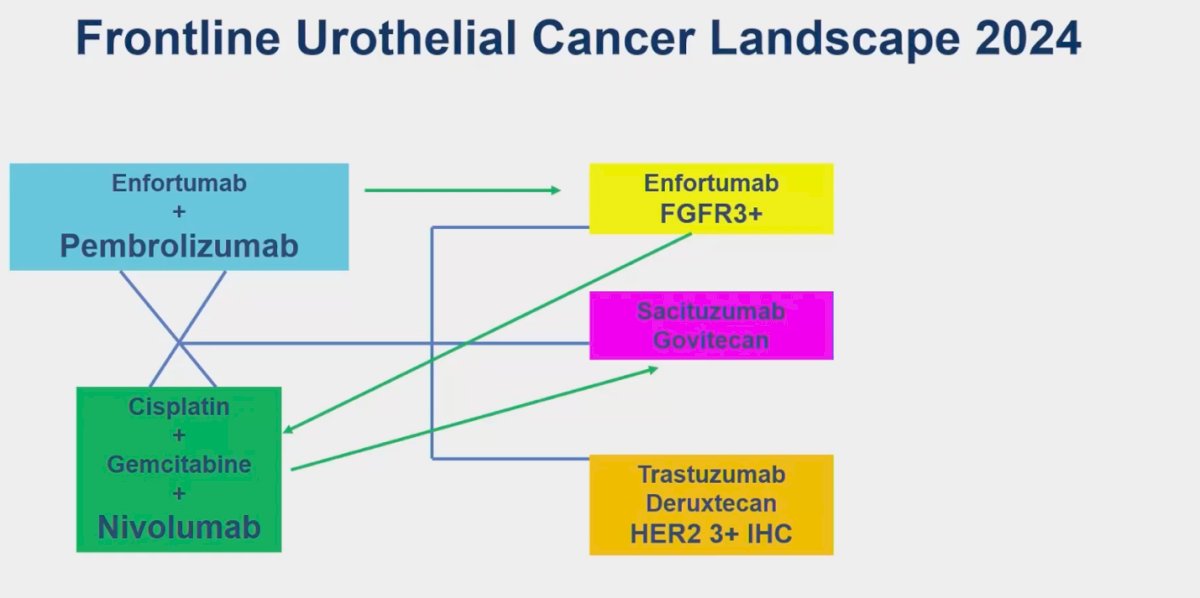
After the presentation of the EV-302 study at ESMO 2023, the frontline therapy for locally advanced unresectable or advanced UC has shifted to EV + pembrolizumab. Ideally, patients who progress after this first line should transition to GC. (2) Another frontline treatment option is nivolumab + GC, as reported in the CheckMate 901 trial. (1) Additional options for patients after progression include erdafitinib, sacituzumab govitecan, and trastuzumab deruxtecan. However, given the numerous available options and tools for improving patient selection, it remains challenging to determine the optimal choice for the first-line setting and after progression.
There are many different variants considered VH for UC. Dr. Hoffman-Censits delved into each one of them separately, which we will discuss below.
Small cell variant histologySmall cell cancer is typically an aggressive tumor, but it often responds well to chemotherapy. Brain involvement is frequently seen at presentation, and recurrence rates are high. The treatment for this histologic variant has been extrapolated from data on small cell lung cancer (SCLC). Etoposide (EP) neoadjuvant chemotherapy (NAC) has been recommended, even for tumors staged as cT1. It has been shown that NAC followed by local treatment improves survival outcomes compared to radical cystectomy (RC) alone. However, any residual small cell cancer at the RC pathology is associated with worse outcomes than residual UC alone. (4)
Data supporting immune checkpoint inhibitors for small cell cancer has been published. However, the preferred approach remains NAC followed by surgery and possibly adjuvant nivolumab. With our current treatment paradigm of using upfront EV + Pembrolizumab for the frontline setting of UC, this approach could be considered and discussed for patients with small cell cancer according to Dr. Hoffman-Censits.
Three clinical trials examining treatment options for the small cell variant are ongoing:
- In the neoadjuvant setting, a trial exploring Atezolizumab with Platinum and Etoposide Chemotherapy Followed by Cystectomy for Patients with Localized Small Cell Neuroendocrine Bladder Cancer (NCT05312671).
- In the first-line setting of advanced extrapulmonary neuroendocrine tumors, a phase II/III trial (NCT05058651) compares the effect of atezolizumab in combination with standard chemotherapy with a platinum drug (cisplatin or carboplatin) and etoposide versus standard therapy alone.
- In the second-line setting, the LASER trial (NCT06228066) is evaluating Lurbinectedin with or without Avelumab in small cell carcinoma of the bladder.
Sarcomatoid differentiated variant histology of urothelial carcinoma (UC) is characterized by malignant spindle cells with a nonspecific morphologic appearance. Retrospective studies generally do not show a significant benefit from NAC or adjuvant chemotherapy (AC), but the receptivity in these studies is low. (5) However, a pooled analysis indicates that NAC can improve overall survival (OS). (6) One specific NAC regimen—cisplatin (35 mg/m²), gemcitabine (800 mg/m²), and docetaxel (35 mg/m²) on days 1 and 8, with G-CSF support every 21 days for four cycles—followed by radical cystectomy (RC) has shown promising results, with a ypCR rate of 38% and a <ypT2 rate of 50%. Additionally, sarcomatoid UC often exhibits high PD-L1 expression, with an overall response rate (ORR) of 35-50% to immune checkpoint inhibitors (ICI) in the first-line setting.
Squamous cell carcinomaSquamous cell carcinoma (SCC) of the urothelial tract is an aggressive variant that can present as locally advanced disease often. One of the notable clinical features is hypercalcemia, which requires careful monitoring of the metabolic panel. Histologically, SCC exhibits keratinization and intercellular bridges, making it indistinguishable from squamous carcinoma of other primary sites.
In a prospective study involving ifosfamide, paclitaxel, and cisplatin, the overall response rate (ORR) was 25% in eight patients with SCC. Data from the SEER database (n=1371) indicates a survival benefit with adjuvant chemotherapy. (7)
SCC shows poorer progression-free survival (PFS) and overall survival (OS) compared to conventional urothelial carcinoma (UC). Specifically, PFS for SCC treated with cisplatin and paclitaxel (CP) was 1.9 months versus 4.8 months for UC (P < .01), and median OS was 9.2 months for SCC compared to 20.7 months for UC (P < .01). Additionally, SCC has a lower ORR to enfortumab vedotin (EV) or EV plus pembrolizumab (EV+P), at 17% versus 70% for UC (P < .01), with a median PFS of 3.4 months for SCC compared to 15.8 months for UC (P < .01). (3)
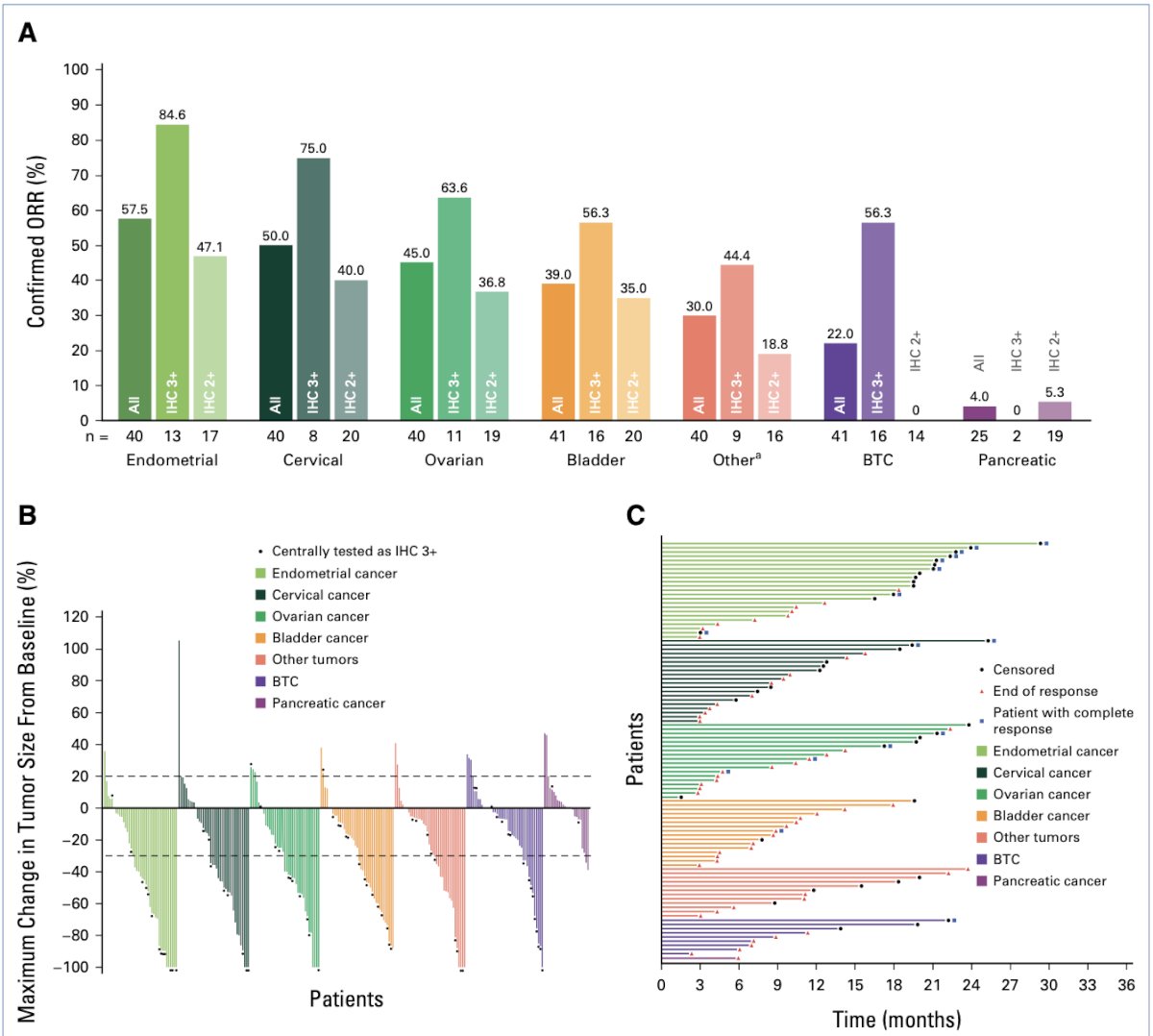
The micropapillary variant of urothelial carcinoma is characterized by HER2 activating alterations. This molecular feature makes it a potential candidate for targeted therapies. In the DESTINY-PanTumor02 Phase II trial, trastuzumab deruxtecan was evaluated in 41 patients with previously treated bladder cancer, including those with micropapillary histology. The overall response rate (ORR) was 56.3% in tumors with IHC3+ HER2 expression and 35% in tumors with IHC2+ expression. These findings highlight the efficacy of HER2-targeted therapy in this aggressive variant and support its use in clinical practice for patients with HER2-positive micropapillary urothelial carcinoma. (8)
Urachal cancer is a rare malignancy accounting for <1% of bladder cancers. This is an aggressive disease with the overall survival for recurrent or metastatic disease currently less than two years. Highlighted at ASCO 2024 was a prospective study (ULTMA; KCSG GU20-03) assessing modified FOLFIRINOX in 21 patients treated between 2021 and 2023. The ORR was 61.9%, with complete responses (CR) in 2 patients (9.5%), partial responses (PR) in 11 patients (52.4%), and stable disease (SD) in 8 patients (38.1%). Progression-free survival (PFS) was 9.3 months, and overall survival (OS) was 19.7 months. (9) Additionally, the MD Anderson Cancer Center (MDACC) retrospective experience presented at ASCO GU 2024 reviewed outcomes with the GemFLP regimen (Gemcitabine, 5-FU, and cisplatin) in 40 patients with urachal cancer, further supporting the efficacy of tailored chemotherapy approaches for this rare and aggressive variant. (10)
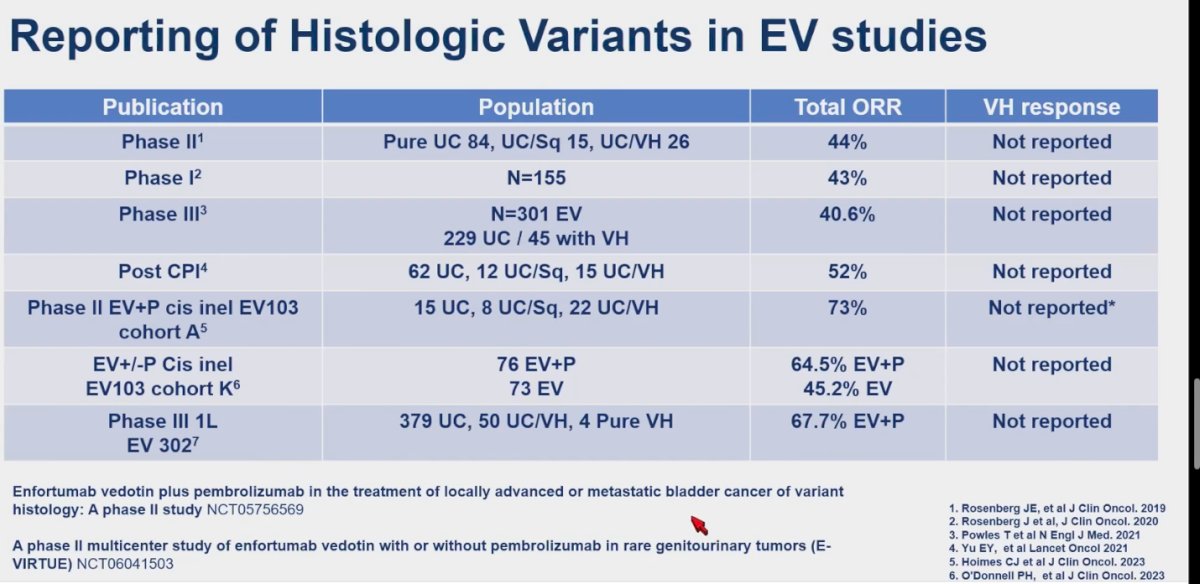
Dr. Hoffman-Censits discussed the application of EV for variant histology urothelial carcinoma , noting that while the population of patients with variant histology was reported in published trials, the response of variant histology to EV was not consistently reported. She highlighted two ongoing studies with immune checkpoint inhibitors (IO) in this context: "Enfortumab vedotin plus pembrolizumab in the treatment of locally advanced or metastatic bladder cancer of variant histology: A phase II study (NCT05756569)" and "A phase II multicenter study of enfortumab vedotin with or without pembrolizumab in rare genitourinary tumors (E-VIRTUE) (NCT06041503) that hopefully would give us some answers into this the treatment of this rare variants.
The UNITE study, a large retrospective cohort, assessing the efficacy of EV in advanced UC including variant histology, included a total of 260 patients treated with EV monotherapy. (n=70), 66 patients with UC and variant histology, and 4 with pure variant histology. The ORR in variant histology was 42% compared to 58% in patients with pure UC (n=142), this was not significant (p=0.056). A detailed response according to different variant histology groups is shown in the table below. (11)
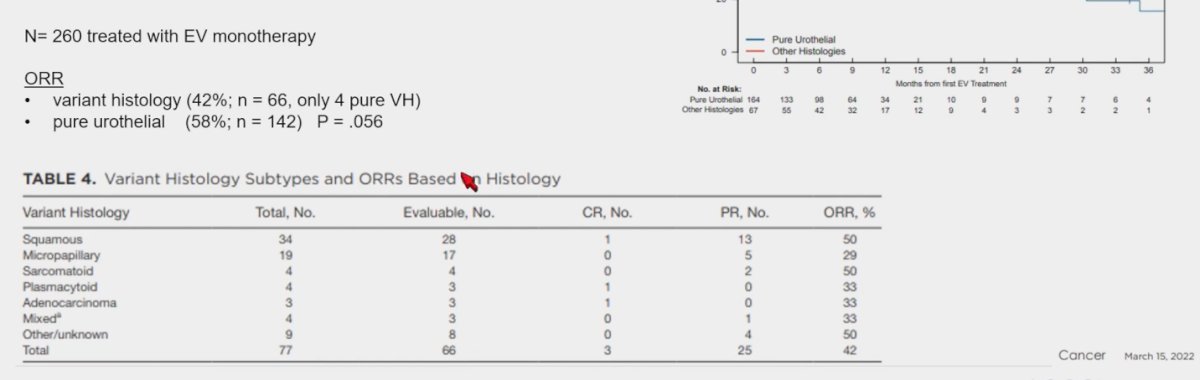
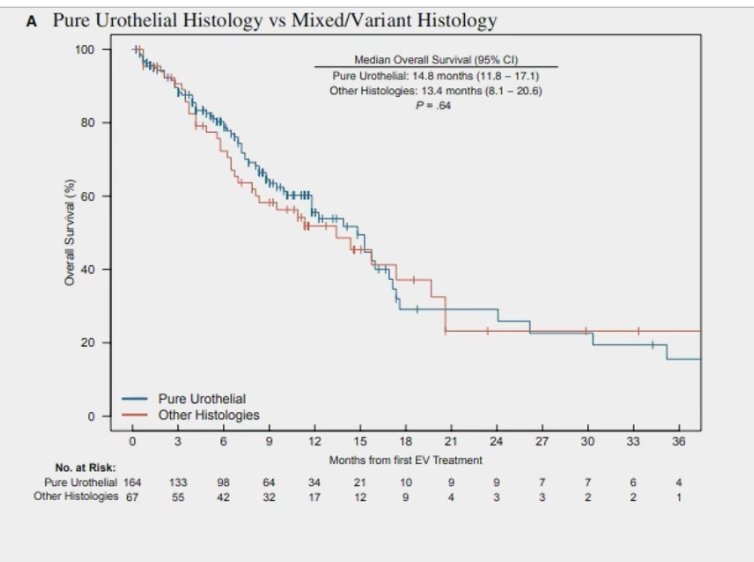
Furthermore, the median OS in pure UC was 14.8 months, compared to 13.4 months (p=0.064) in patients treated with EV monotherapy.
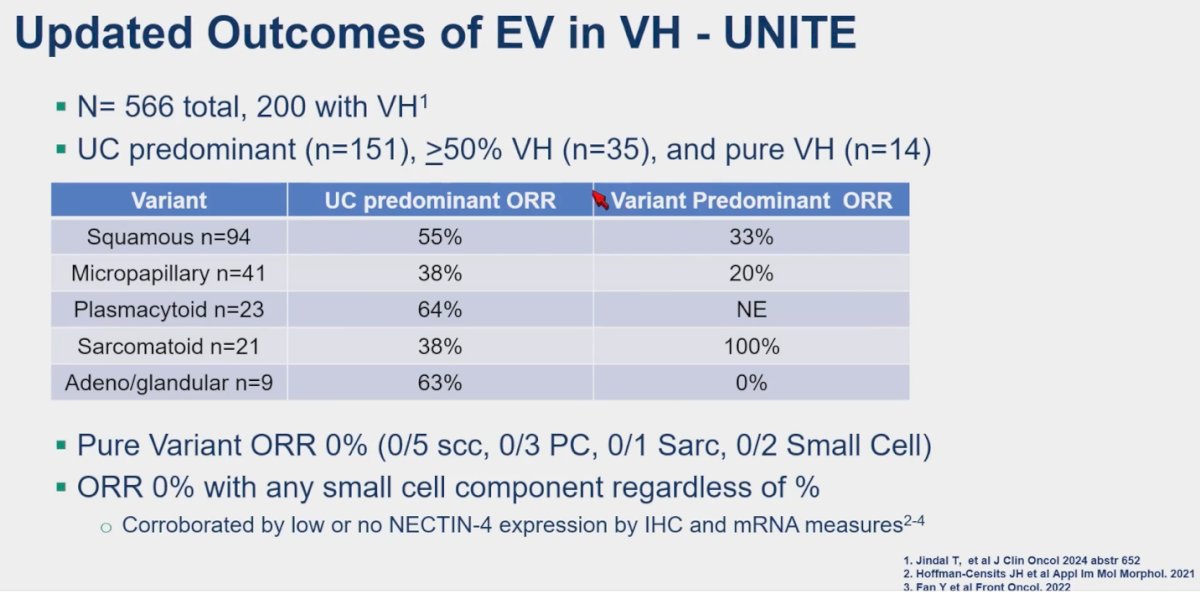
Dr. Hoffman-Censits presented updated outcomes from the UNITE study, now encompassing a total of 566 patients, including 200 with variant histology. Among these, UC predominant tumors (n=151), those with ≥50% variant histology (n=35), and pure variant histology cases (n=14) were analyzed. The study revealed a 0% ORR in pure variant histology, regardless of subtype (0/5 SCC, 0/3 PC, 0/1 Sarc, 0/2 Small Cell), as well as no response observed with any small cell component, irrespective of percentage.
In the UNITE study, outcomes were evaluated for 116 patients treated with Sacituzumab Govitecan, with 44 of them having variant histology. The overall response rate (ORR) for all patients was 24%, comparable to 23% for those with any variant histology. When analyzed based on specific VH groups, the results are detailed in the table below.

SMART is an open-label, non-randomized Phase 2 trial designed to evaluate treatment outcomes in patients with locally advanced (unresectable) or metastatic genitourinary (GU) tumors of specific histologies, including small cell carcinoma, squamous cell carcinoma, primary adenocarcinoma of the bladder or urinary tract, renal medullary carcinoma (RMC), or squamous cell carcinoma of the penis. Patients will receive Sacituzumab Govitecan (SG) or SG and concomitant atezolizumab, results are awaited. (11)
Of note, the CheckMate 901 study included a significant proportion of patients with variant histology, comprising 51% in the Nivolumab + GC arm and 53% in the GC arm. However, the outcomes for this specific population have not yet been reported. (1)

With our new treatment paradigms, patients with variant histology in 2024 should be approached carefully. Dr. Hoffman-Censits suggests initiating treatment with platinum-based chemotherapy. For those with biomarker-positive tumors, she advocates for prioritizing other drugs as the initial treatment option.

These are the key takeaways Dr. Hoffman-Censits used to conclude her presentation:
- Prospective trials face challenges in funding and accrual, requiring larger sample sizes for meaningful results.
- Basket studies offer opportunities but have limited capacity for extensive clinical development.
- Reporting practices of variant histology in large prospective studies vary, potentially impacting data interpretation.
- Historical practices of excluding subtypes/women/African American may have contributed to the perception of bladder cancer as predominantly affecting white males, highlighting the need for more inclusive trial design.
- There are now two potent frontline regimens available for bladder cancer, raising questions about how to maximize their combined benefits.
- Biomarker-selected and unselected agents approved for second-line use in urothelial carcinoma (UC) provide an expanding toolkit for treating non-UC subtypes.
- Biopsying at disease progression is recommended to inform treatment sequencing and target specific subtypes, particularly in variant histologies.
- The "rainbow" of available treatments presents challenges for FDA approval in data-scarce areas like micropapillary and small cell carcinoma.
Presented By: Jean H. Hoffman-Censits, MD, Genitourinary medical oncologist at The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, and The Greenberg Bladder Cancer Institute at Johns Hopkins.
Written By: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References:
- van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, Bedke J, Oldenburg J, Chatta G, Ürün Y, Ye D, He Z, Valderrama BP, Ku JH, Tomita Y, Filian J, Wang L, Purcea D, Patel MY, Nasroulah F, Galsky MD; CheckMate 901 Trial Investigators. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789. doi: 10.1056/NEJMoa2309863. Epub 2023 Oct 22. PMID: 37870949.
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675
- Koehne EL, Bakaloudi DR, Ghali F, Nyame Y, Schade GR, Grivas P, Yezefski TA, Hawley JE, Yu EY, Hsieh AC, Montgomery RB, Psutka SP, Gore JL, Wright JL. Adjuvant Chemotherapy and Survival After Radical Cystectomy in Histologic Subtype Bladder Cancer. Clin Genitourin Cancer. 2024 Apr 25:102100. doi: 10.1016/j.clgc.2024.102100. Epub ahead of print. PMID: 38763862.
- Hoffman-Censits J, Choi W, Pal S, Trabulsi E, Kelly WK, Hahn NM, McConkey D, Comperat E, Matoso A, Cussenot O, Cancel-Tassin G, Fong MHY, Ross J, Madison R, Ali S. Urothelial Cancers with Small Cell Variant Histology Have Confirmed High Tumor Mutational Burden, Frequent TP53 and RB Mutations, and a Unique Gene Expression Profile. Eur Urol Oncol. 2021 Apr;4(2):297-300. doi: 10.1016/j.euo.2019.12.002. Epub 2020 Feb 13. PMID: 32061548.
- Vetterlein MW, Wankowicz SAM, Seisen T, Lander R, Löppenberg B, Chun FK, Menon M, Sun M, Barletta JA, Choueiri TK, Bellmunt J, Trinh QD, Preston MA. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer. 2017 Nov 15;123(22):4346-4355. doi: 10.1002/cncr.30907. Epub 2017 Jul 25. PMID: 28743155.
- Almassi N, Vertosick EA, Sjoberg DD, Wong NC, Huang C, Pietzak EJ, Cha EK, Donahue TF, Dalbagni G, Bochner BH, Iyer G, Rosenberg JE, Bajorin DF, Al-Ahmadie H, Goh AC. Pathological and oncological outcomes in patients with sarcomatoid differentiation undergoing cystectomy. BJU Int. 2022 Apr;129(4):463-469. doi: 10.1111/bju.15428. Epub 2021 May 24. PMID: 33866683; PMCID: PMC8522172.
- Abdel-Rahman O. Squamous Cell Carcinoma of the Bladder: A SEER Database Analysis. Clin Genitourin Cancer. 2017 Jun;15(3):e463-e468. doi: 10.1016/j.clgc.2016.10.007. Epub 2016 Oct 28. PMID: 27876505.
- Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, Jung KH, Ługowska I, Manso L, Manzano A, Melichar B, Siena S, Stroyakovskiy D, Fielding A, Ma Y, Puvvada S, Shire N, Lee JY. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 2024 Jan 1;42(1):47-58. doi: 10.1200/JCO.23.02005. Epub 2023 Oct 23. PMID: 37870536; PMCID: PMC10730032.
- Lee, JL et al A multicenter phase II study of modified FOLFIRINOX for first-line treatment for advancedurachal cancer (ULTMA; KCSG GU20-03).J Clin Oncol 42, 2024 (suppl 16; abstr 4510)
- Moussa MJ et al. Clinical outcomes of frontline GemFLP in advanced urachal and non-urachal adenocarcinomas of the urinary tract: The MD Anderson Cancer Center (MDACC) experience. J Clin Oncol Abstr 630
- Koshkin VS, Henderson N, James M, Natesan D, Freeman D, Nizam A, Su CT, Khaki AR, Osterman CK, Glover MJ, Chiang R, Makrakis D, Talukder R, Lemke E, Olsen TA, Jain J, Jang A, Ali A, Jindal T, Chou J, Friedlander TW, Hoimes C, Basu A, Zakharia Y, Barata PC, Bilen MA, Emamekhoo H, Davis NB, Shah SA, Milowsky MI, Gupta S, Campbell MT, Grivas P, Sonpavde GP, Kilari D, Alva AS. Efficacy of enfortumab vedotin in advanced urothelial cancer: Analysis from the Urothelial Cancer Network to Investigate Therapeutic Experiences (UNITE) study. Cancer. 2022 Mar 15;128(6):1194-1205. doi: 10.1002/cncr.34057. Epub 2021 Dec 9. PMID: 34882781.
- Andre Rashad Kydd et al.SMART: A phase II study of sacituzumab govitecan (SG) with or without atezolizumab immunotherapy in rare genitourinary (GU) tumors such as small cell, adenocarcinoma, and squamous cell bladder/urinary tract cancer, renal medullary carcinoma (RMC) and penile cancer.. JCO 42, TPS4627-TPS4627(2024).DOI:10.1200/JCO.2024.42.16_suppl.TPS4627


