(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a kidney and bladder rapid oral abstract session. Dr. Zhigong Wei presented the results of a single open-labeled, phase II trial evaluating the combination of camrelizumab plus apatinib for patients with previously treated, advanced adrenocortical carcinoma.
Adrenocortical carcinoma is a rare, aggressive malignancy with limited treatment options and associated with poor prognoses. Currently, chemotherapy plus mitotane remains the standard of care 1st line therapy; however, responses remain poor with an objective response rate of 23% and a median progression-free survival of 5 months.1 Therapeutic options are further limited for patients with advanced adrenocortical carcinoma who have failed 1st line therapy. The efficacy of immune checkpoint inhibitor monotherapy in the 2nd line setting is also poor with response rates of 10 to 14%.
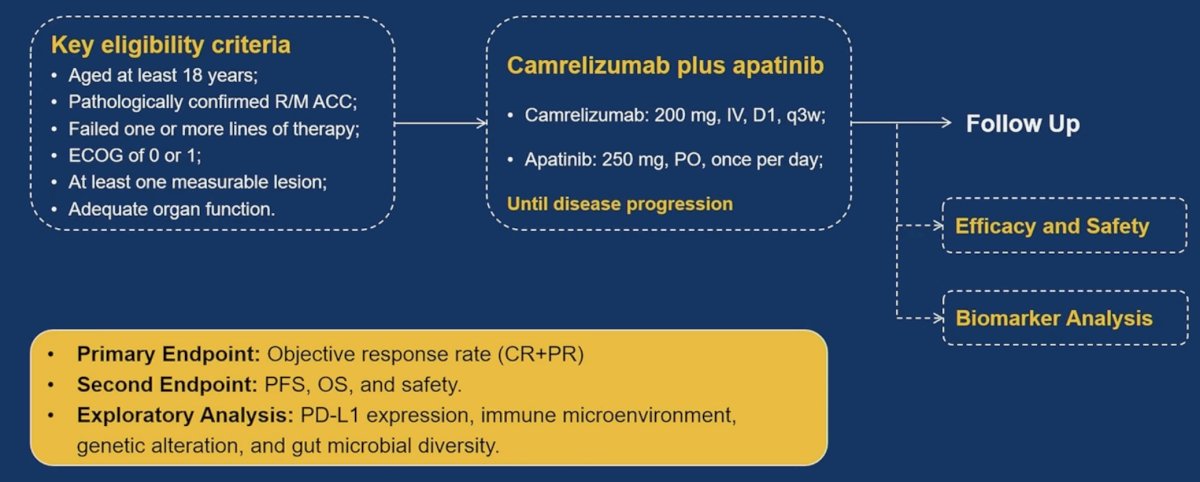
The objective of this study was to assess the activity and safety of camrelizumab, a PD-1 inhibitor, plus apatinib, a VEGFR2-targeted small molecular TKI, as combination therapy in patients with advanced adrenocortical carcinoma who have failed one or more lines of therapy. The study design and key eligibility criteria are summarized below. Patients in this trial received camrelizumab 200 mg intravenously every three weeks plus apatinib 250 mg orally once daily until disease progression. The primary study endpoint was objective response rate, defined as a partial or complete response. The secondary/exploratory endpoints included:
- Progression-free survival
- Overall survival
- Safety
- PD-L1 expression
- Immune microenvironment
- Genetic alterations
- Gut microbial diversity
This trial included 21 patients who were allocated to and received the intervention. Of these 21, 13 discontinued the intervention, most commonly due to disease progression, and eight patients had either finished intervention (n=1) or remains on treatment at data cutoff (n=1).
From an efficacy standpoint, the objective response rate was 52% in the patient cohort (all partial responses). Stable disease was observed in an additional 43%. There was one patient with progressive disease as best response.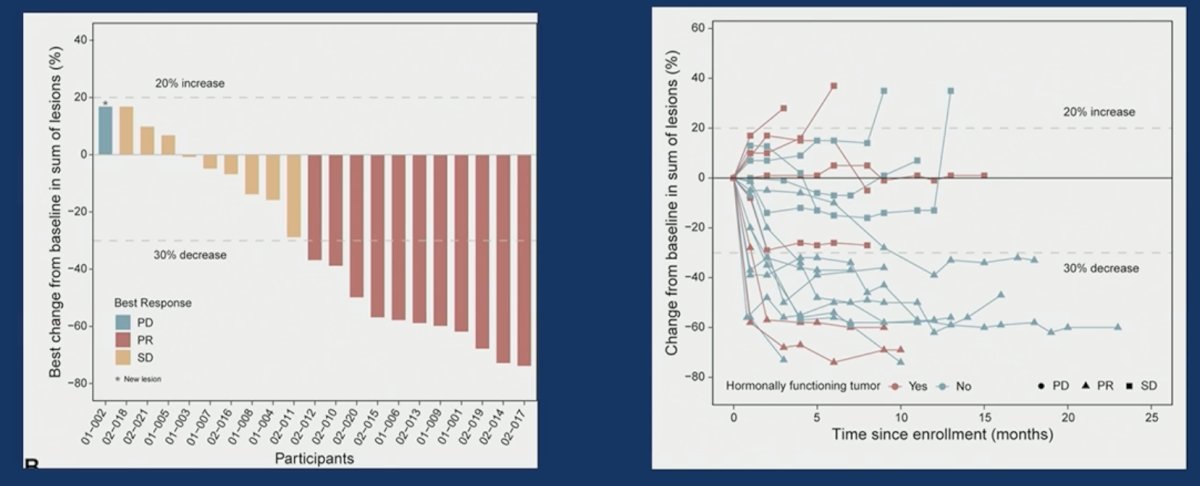
The median progression-free survival was 12.6 months, and the median overall survival was 20.9 months. The corresponding 1-year rates were 57.1% and 74.5%, respectively.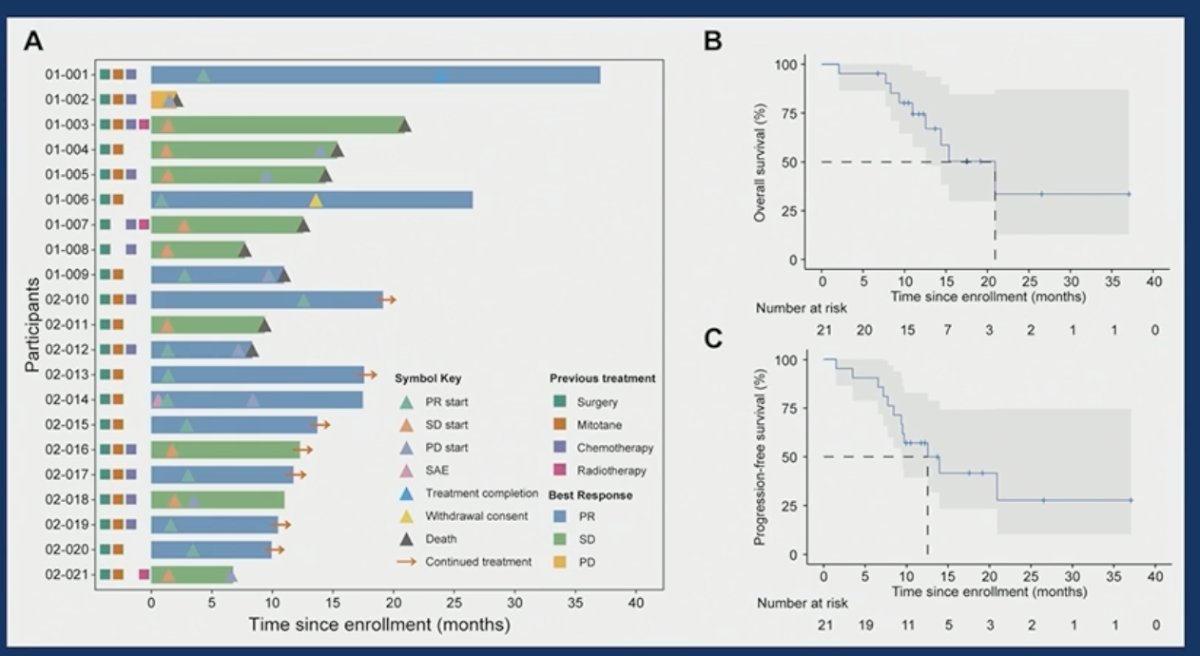
From a safety standpoint, the most common grade 3–4 treatment-related adverse events were hepatic enzymes (ALT/AST) elevations (24%) and lymphopenia (19%).
Correlative analysis demonstrated that none of CD4+, CD8+, nor CD56+ tumor cell levels was related to treatment response.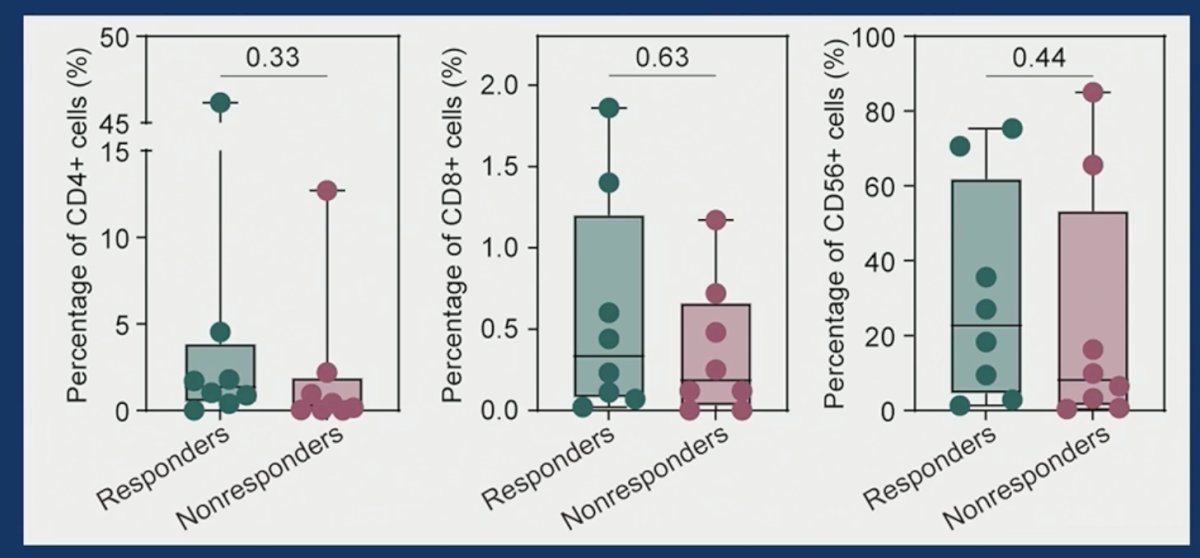
Higher CD8+ T cell percentage at baseline was associated with an improved likelihood of response to combination therapy. Peripheral blood CD4+/8+ T cell abundance was associated with treatment response.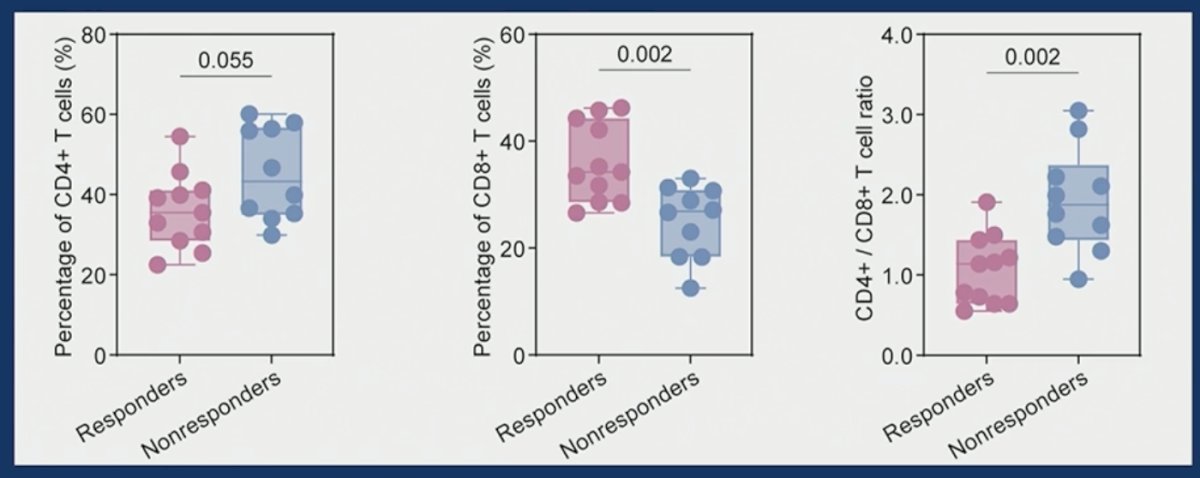
Only two patients (10%) were PD-L1 positive with a CPS of 21; one experienced stable disease and the other had a partial response. No specific somatic alteration was correlated with the observed response.
Notable limitations to this study include a small sample size and the absence of a control arm. The absence of post-treatment tumor tissue limited the ability to correlate treatment response with changes in the tumor microenvironment.
Dr. Wei concluded as follows:
- Camrelizumab and apatinib combination therapy for adrenocortical carcinoma patients with disease progression following ≥1 line of therapy is associated with an objective response rate of 52%, which is higher than what has been historically reported in this setting.
- Peripheral blood CD4+/8+ T cell abundance was associated with treatment response.
- The potential of camrelizumab plus apatinib deserves further evaluation in this setting.
Presented by: Zhigong Wei, MD, Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:- Fassnacht M, Terzolo M, Allolio B, et al. Combination Chemotherapy in Advanced Adrenocortical Carcinoma. N Engl J Med. 2012;366: 2189-97.


