(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a kidney and bladder rapid oral abstract session. Dr. Sumanta Pal presented the preliminary safety, pharmacokinetics, and clinical activity of DFF332, an oral HIF2α inhibitor, as monotherapy in a phase I dose-escalation study in patients with advanced clear cell renal cell carcinoma (ccRCC).
ccRCC is the most common subtype of RCC, accounting for 75% to 85% of all RCC cases. Despite a considerable improvement in outcomes of patients with metastatic ccRCC, novel agents targeting alternative mechanisms are needed for patients who have progressed on standard-of-care therapies (VEGF-directed agents and immune checkpoint inhibitors). HIF-2α, an oncogenic driver in ccRCC, is overexpressed and plays a critical role in promoting tumor growth and progression in ccRCC. Targeting HIF-2α inhibition has been proven to be an effective therapeutic option in patients with advanced ccRCC. DFF332, a small molecule inhibitor that selectively targets HIF-2α transcriptional activity, has shown dose-dependent antitumor efficacy and good tolerability in pre-clinical models of ccRCC.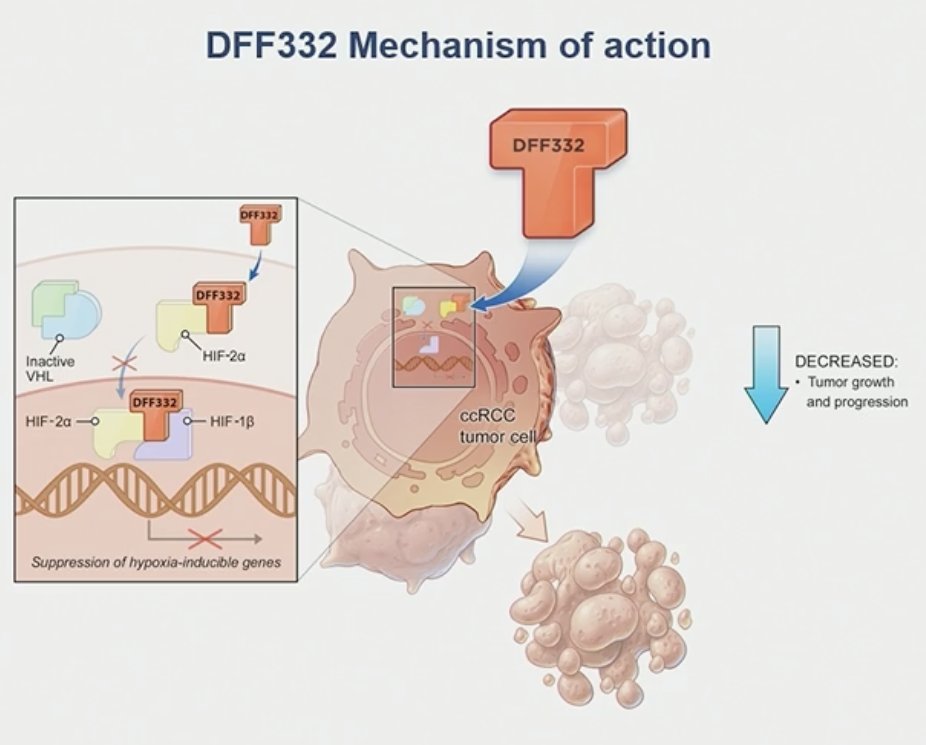
This was a phase I/1b, first-in-human, open-label, multicenter study of DFF332 monotherapy that included patients meeting the following eligibility criteria:
- Aged ≥18 years
- ECOG performance status ≤1
- Histologically confirmed unresectable, locally advanced, or metastatic ccRCC
- Disease progression following any standard of care therapy, including PD-1/ PD L1 checkpoint inhibitor and a VEGF-targeted therapy as monotherapy or in combination
- Measurable disease, RECIST v1.1
- Tumor amenable to biopsy
The dose escalation schedule is illustrated below, starting with a once-weekly regimen (50 to 100 mg QW) and increasing the frequency to once daily at doses of 25 to 150 mg. The primary study objective was safety and tolerability, with secondary objectives of assessing pharmacokinetics and anti-tumor activity.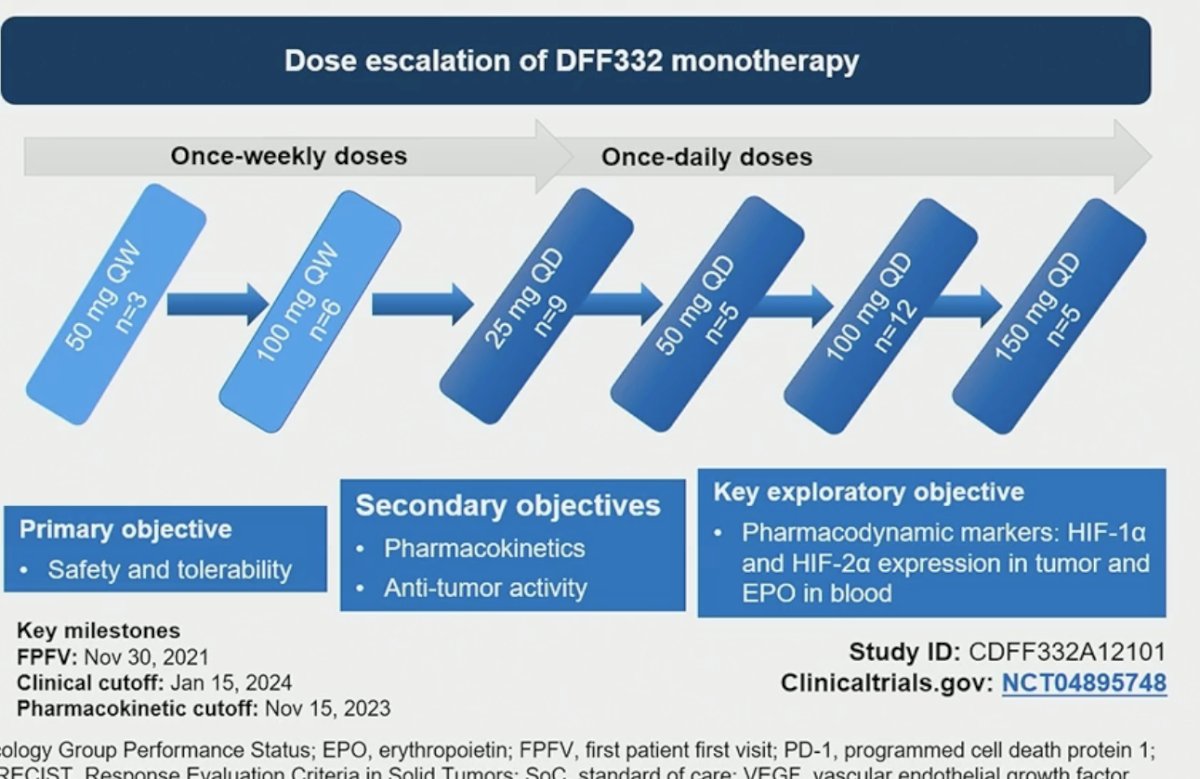
The study cohort included 40 patients. The median patient age at inclusion was 62.5 years. Patients most commonly had IMDC favorable or intermediate-risk disease at baseline (87.5%). This was a heavily pre-treated cohort with 87.5% of patients having received ≥2 prior lines of therapy.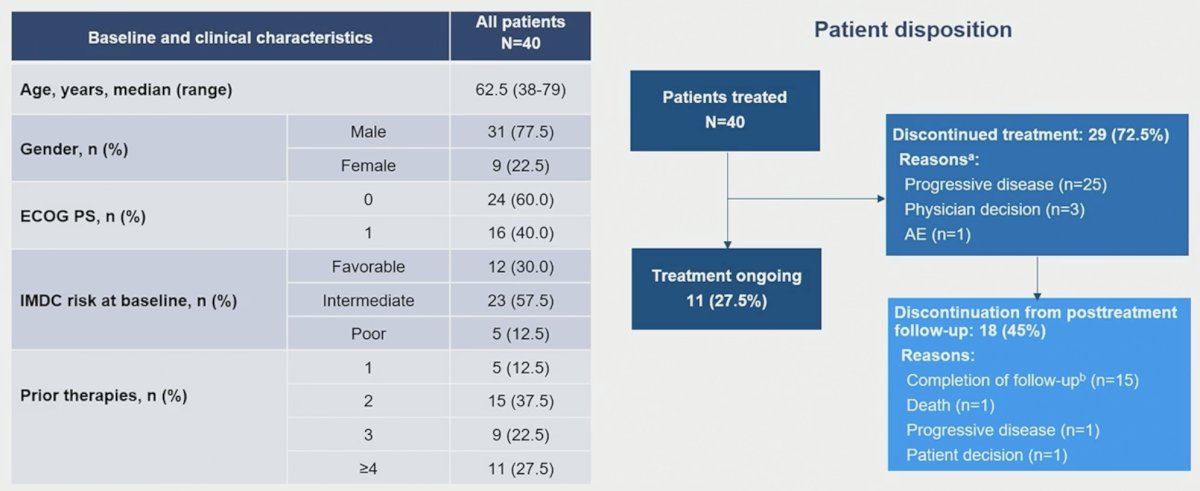
The disease control rate was 52.5% with a best response of partial response in two patients (5%) and stable disease in 19 patients (47.5%).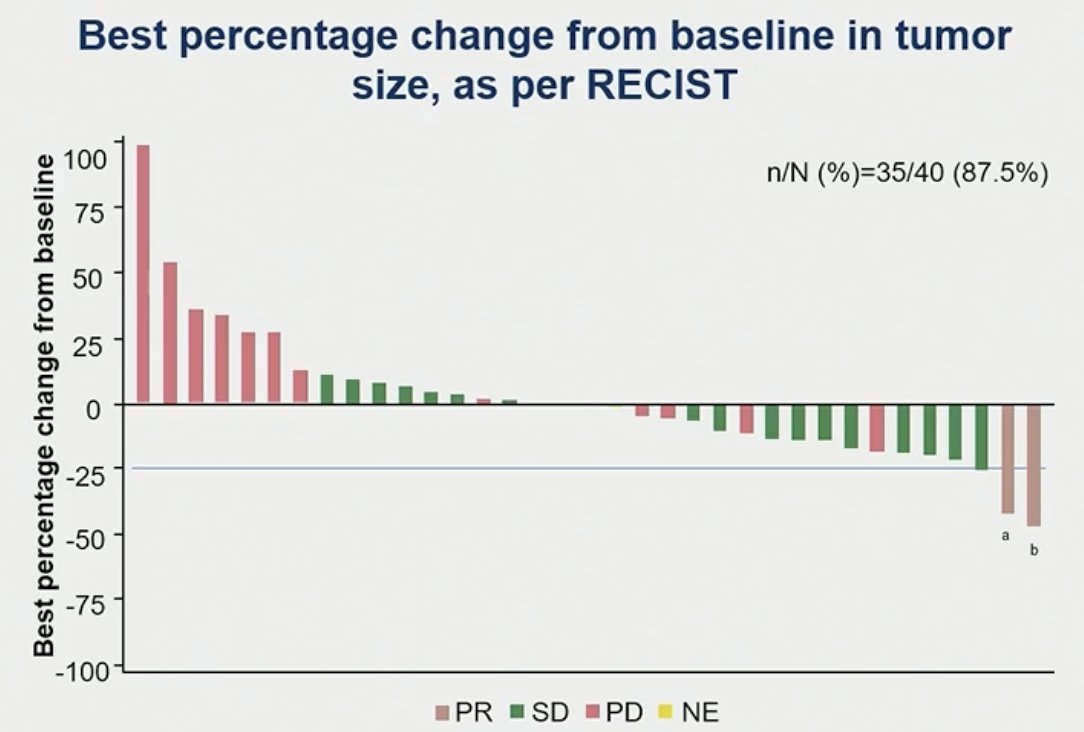
At cut-off, treatment was ongoing in 11 patients (27.5%) with median (range) duration of exposure of 17.9 (1.0–75.6) weeks.
From a pharmacokinetic standpoint, DFF332 demonstrated:
- Fast oral absorption (median Tmax ~ 1–2 hours)
- Slow elimination, effective half-life ~ 85 days (determined by population pharmacokinetic analysis)
- Nearly dose-proportional exposure (AUC and Cmax) with daily or weekly dosing
- Mean AUC0-24h 10.1-fold higher on C2D1 versus C1D1 at 100 mg once daily

Most of the adverse events were grade 1–2 in severity and no drug-limiting toxicities were reported. Adverse events, regardless of the study treatment, were reported in 38 patients (95%). The most common adverse events were fatigue (37.5%) and anemia (32.5%). Treatment-related adverse events of any grade were reported in 25 patients (62.5%). The most common such events were anemia, fatigue, and hypercholesterolemia (grade <3, each). Two patients reported treatment-related dyspnea, and one reported a treatment-related serious adverse event (grade 3 hypertension).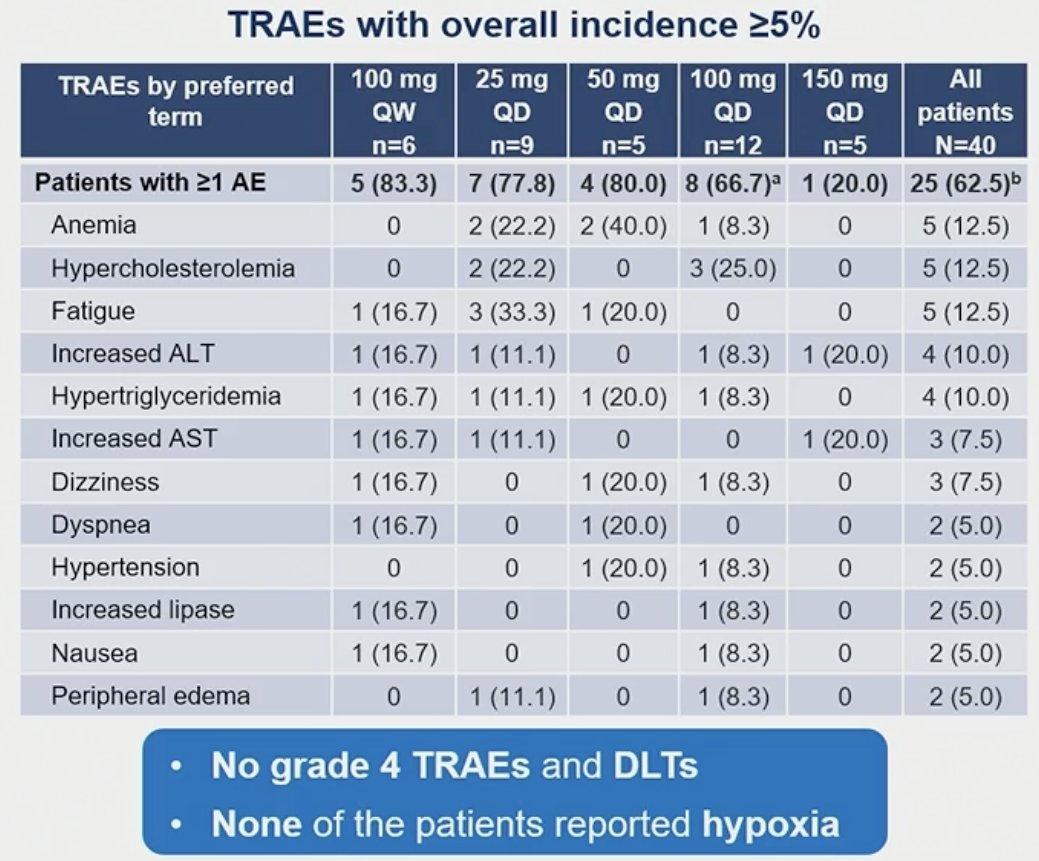
Baseline expression of HIF-2α and HIF-1α were not correlated, and they were not correlated with response.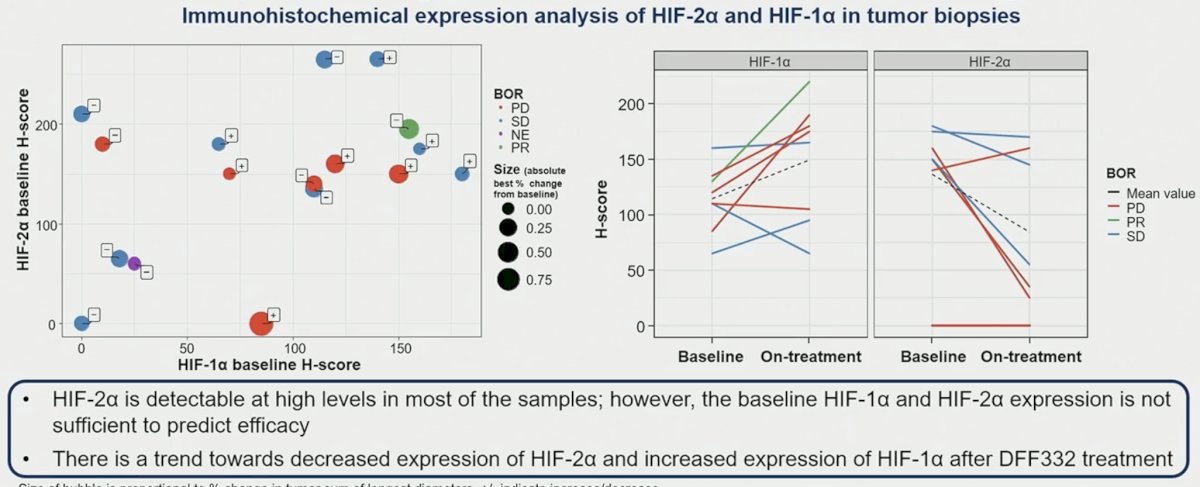
DFF332 was associated with dose-dependent pathway modulation of plasma erythropoietin levels.
Dr. Pal concluded as follows:
- During this first in-human phase I study, DFF332 monotherapy was well tolerated and shown to be safe across all doses and schedules with no dose-limiting toxicities or treatment-related adverse events of grade ≥3 in ccRCC patients.
- At data cutoff, of 40 patients who had been treated, 11 (27.5%) were still ongoing in the study.
- Initial antitumor activity is observed at once-daily doses:
- Two patients (5%) had objective responses achieving confirmed partial responses (1 patient in the 25 mg QD group and 1 patient in the 100 mg QD group) and 19 patients (47.5%) with stable disease.
- There is no obvious correlation between HIF-2α and HIF-1α baseline expression and with response to treatment.
- Dose-dependent pathway modulation of plasma erythropoietin levels was seen.
- The study was halted before an optimal dose could be identified, preventing the realization of the full potential of DFF332. However, if favorable safety and efficacy are confirmed in a larger series following dose optimization, DFF332 could be conducive to effective combination strategies.
Presented by: Sumanta K. Pal, MD, FASCO, Professor, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024


