(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a diagnostic and therapeutic strategies in renal cell carcinoma (RCC) state-of-the-art session. Dr. Yu-Wei Chen discussed:
- The current evidence supporting sequencing strategies in metastatic RCC
- Management of treatment-related medication and financial toxicities
- Early integration of palliative care in kidney cancer care
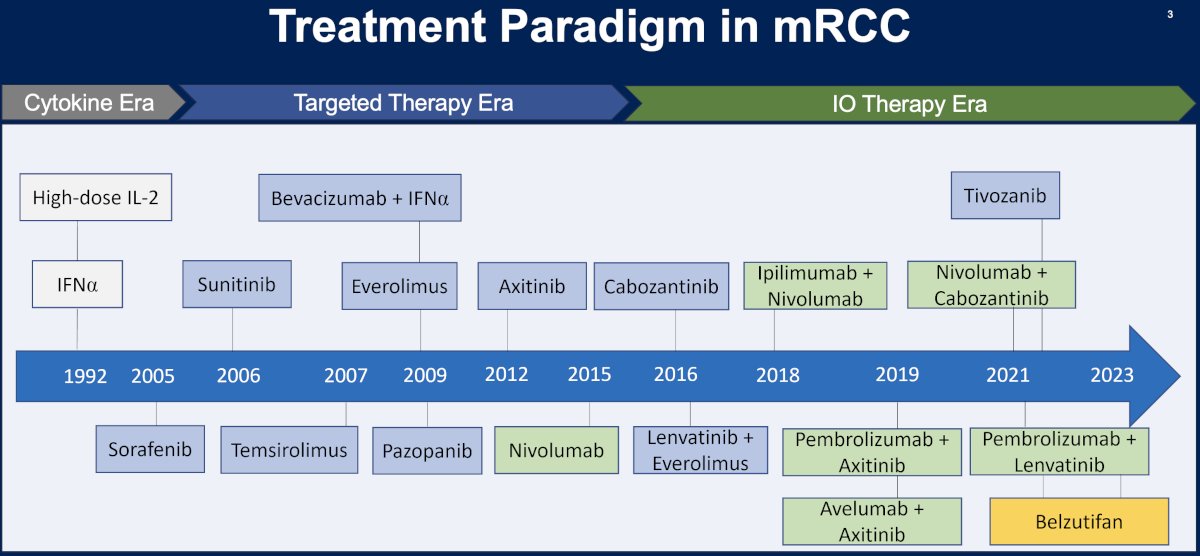
The treatment paradigm for metastatic RCC has significantly evolved over the last three decades, transitioning from the cytokine era (1992 – 2005) to the targeted therapy ear (2005 – 2016) to the immunotherapy era, with the first immunotherapy combination approved by the FDA in April 2018 (ipilimumab + nivolumab).
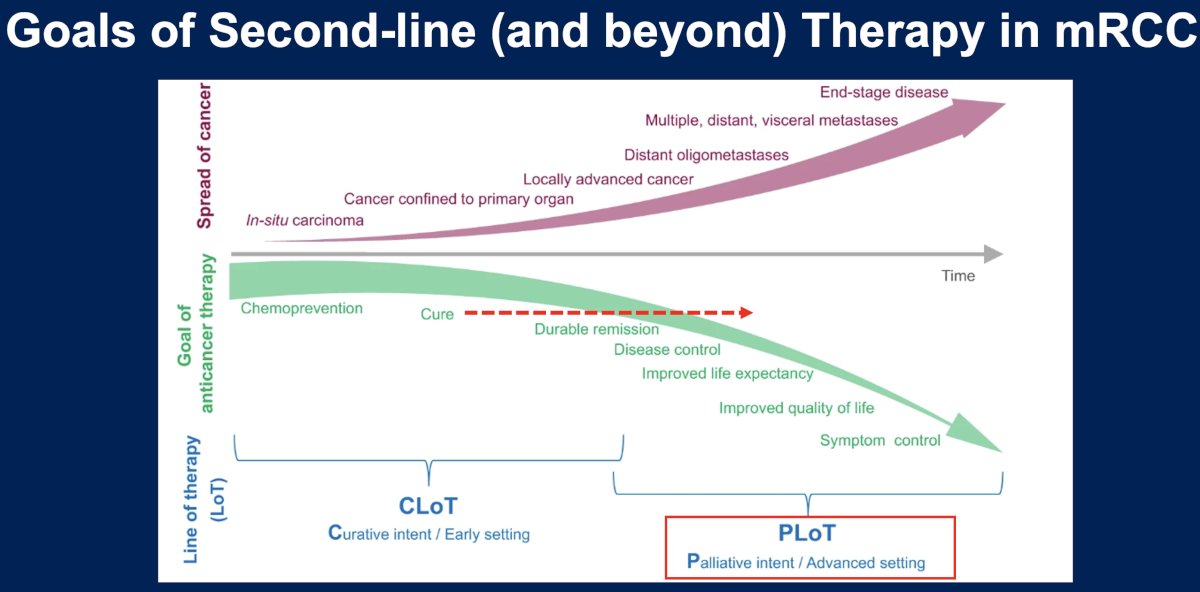
What are the goals of ≥ 2nd line therapy in metastatic RCC? While initial lines of therapy have a curative intent, later lines of therapy have the goals of:
- Disease control
- Improving life expectancy
- Improving quality of life
- Symptom control
This is in contrast to earlier lines of therapy which have the goals of potential chemoprevention and cure with durable remissions.
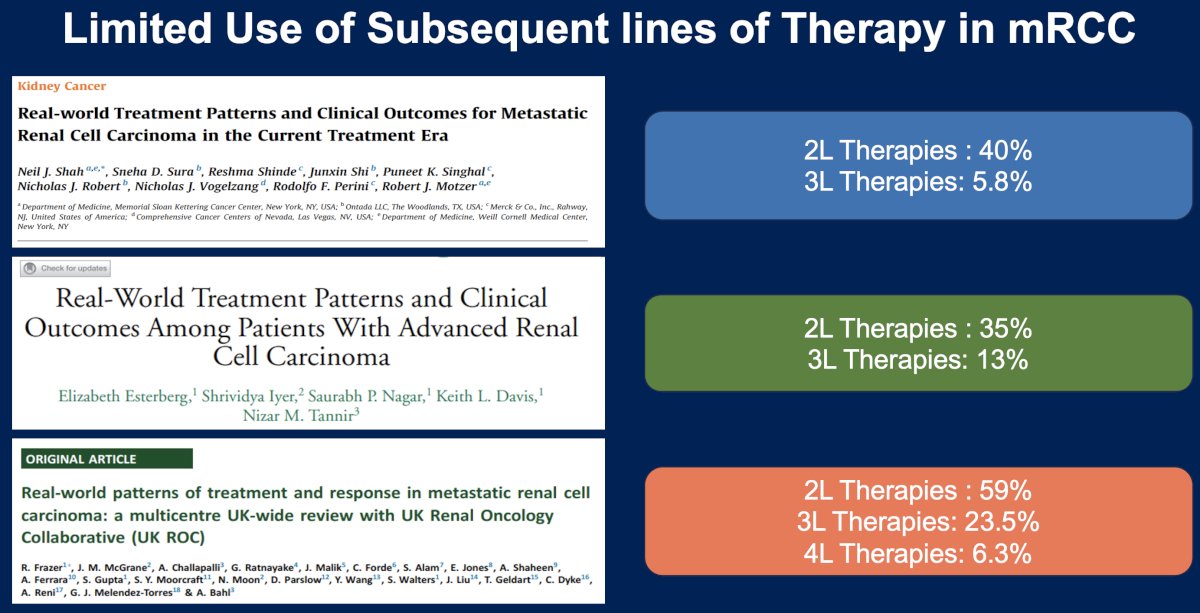
While there are numerous options emerging for the treatment of RCC patients in later line settings, the use of subsequent lines of therapy in metastatic RCC remains underwhelming in the real-world setting. As summarized in the slide below, the use of 2nd line therapies ranges between 35% and 59%, while the use of third line therapies ranges between 6% and 24%.1-3
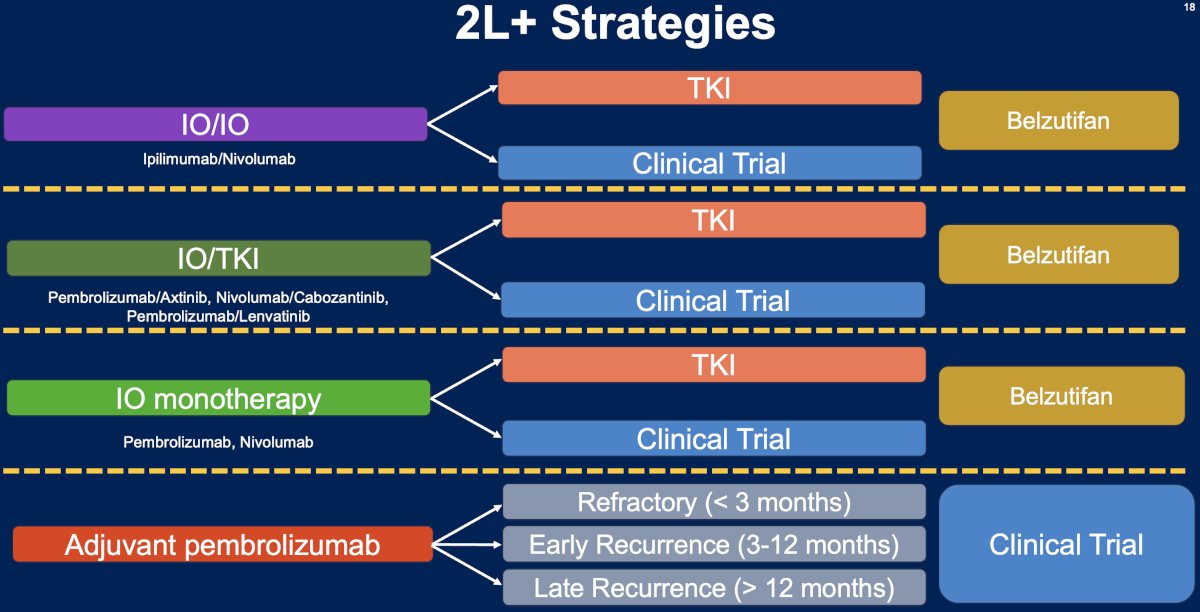
What are the potential 2nd line treatment strategies for metastatic RCC patients? These strategies are strongly contingent upon the initial treatment received in the 1st line setting (summarized in the figure below). The underlying principle here is that patients receive an alternative agent(s) with a different mechanism of action that minimizes the odds of cross resistance.
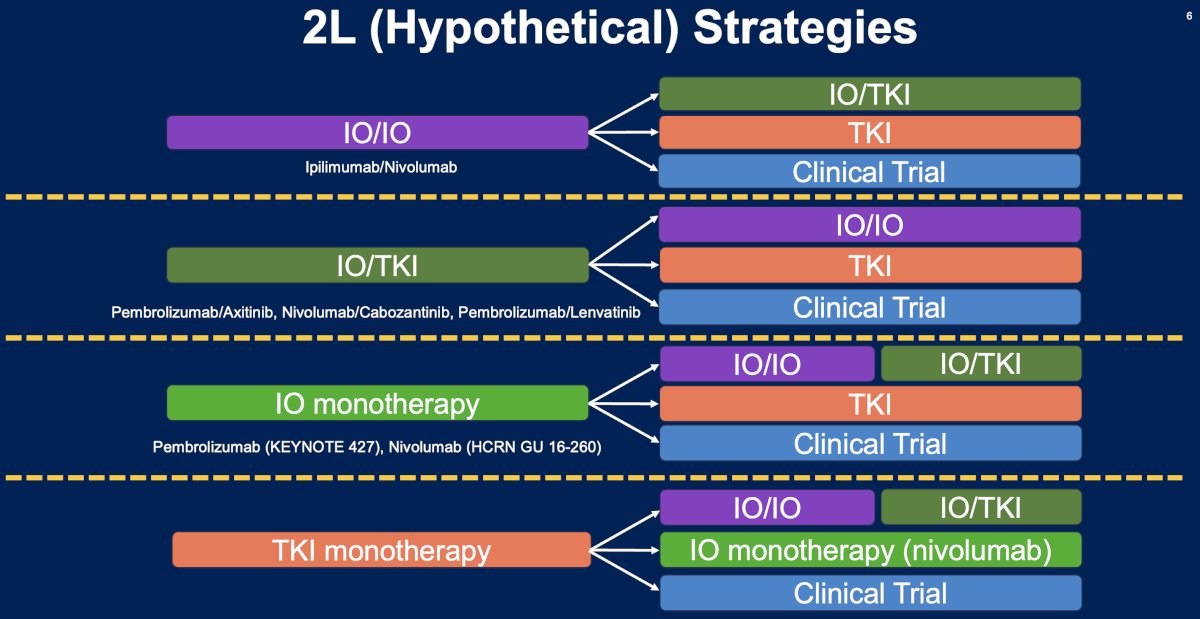
Currently, the evidence for specific 2nd line treatment strategies remains weak. This is evidenced by the NCCN recommendations for the treatment of patients with relapsed or stage IV kidney cancer. There are currently no ‘preferred regimens’ for patients who are either immune-oncology (IO) therapy-naïve or who have received prior IO therapy. The ‘other recommended regimens’ by the NCCN are strongly influenced by prior therapy received.

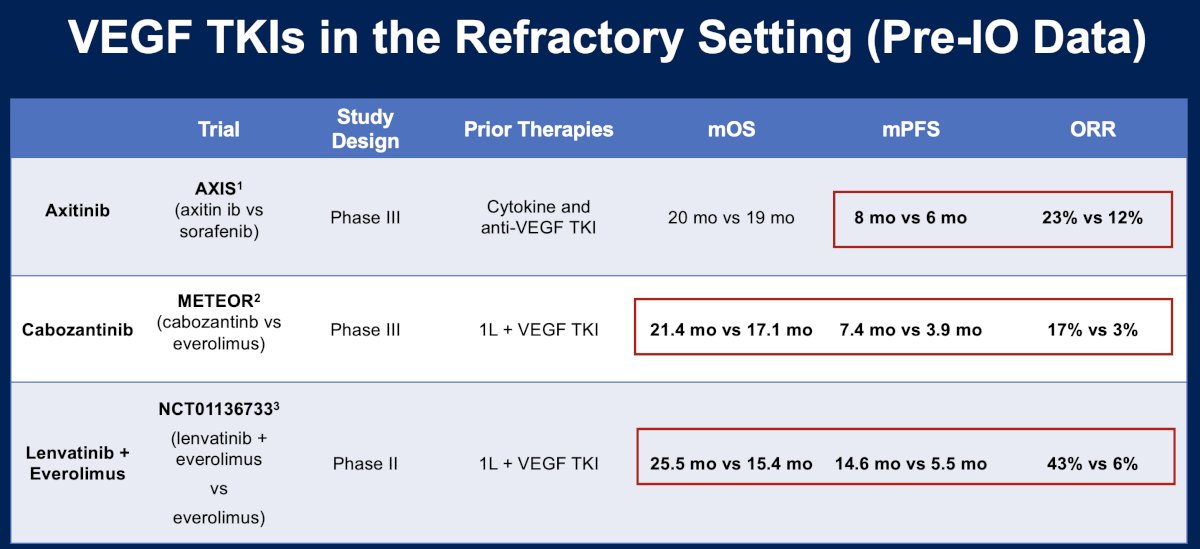
There have been numerous trials that have evaluated vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors (TKIs) in the pre-IO era treatment refractory setting. AXIS was a phase 3 trial that randomized 723 patients with metastatic clear cell RCC that progressed despite first-line therapy containing sunitinib, bevacizumab plus interferon-alfa, temsirolimus, or cytokines to either axitinib or sorafenib.4 METEOR was a phase 3 trial that similarly randomized such patients to either cabozantinib or everolimus.5 In 2015, Motzer et al. published the results of a phase II trial that compared the combination of lenvatinib + everolimus to single agent everolimus in patients who had received VEGF TKIs in the 1st line setting.6 Overall, the median overall survival for these patients was approximately two years. However, median progression-free survival and overall response rates appeared to strongly favor the combination of lenvatinib + everolimus (14.6 months and 43%, respectively), compared to the other options evaluated in this setting.
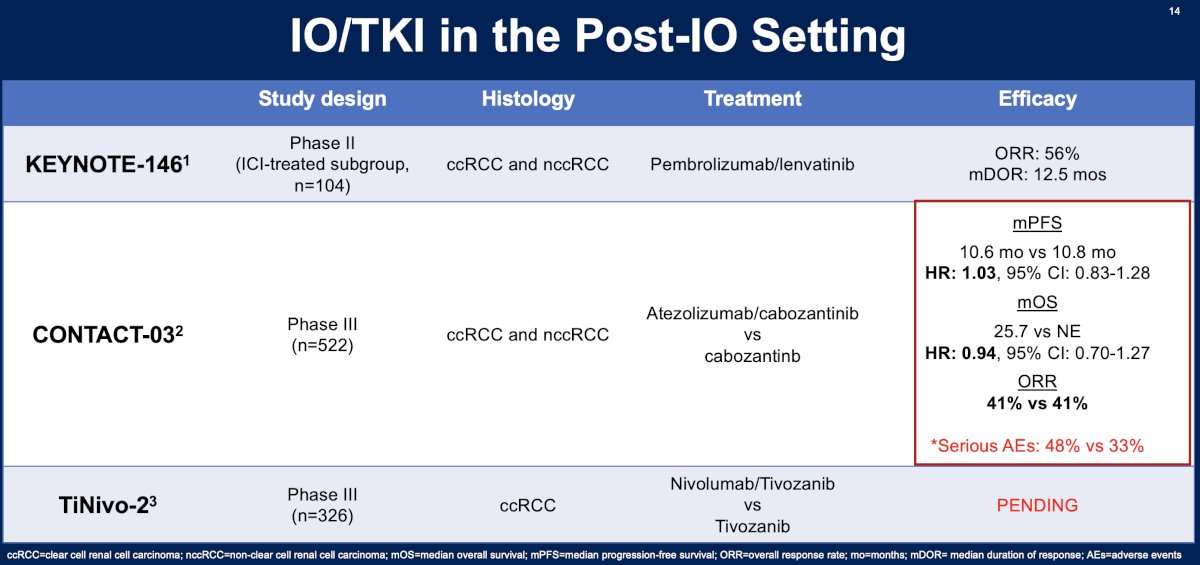
One of the most studied agents in the post-IO setting is cabozantinib. As summarized in the table below, this agent has been evaluated in this setting in four trials: BREAKPOINT, CaboPoint, CANTANA, and CONTACT-03. Overall, the objective response rates with cabozantinib ranged between 25% and 41%.7,8

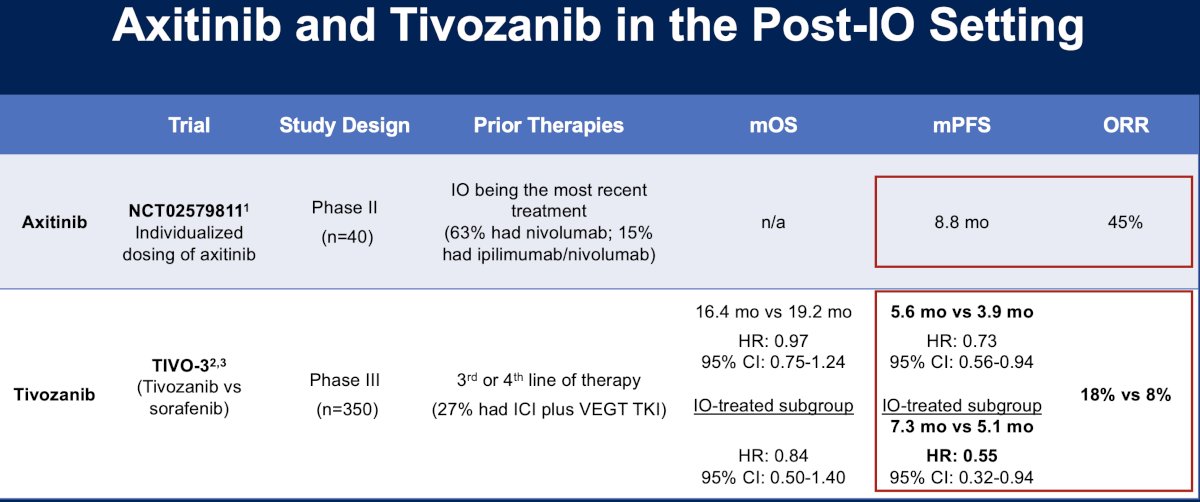
Other agents to be evaluated in the post-IO setting include axitinib (NCT02579811)9 and tivozanib (TIVO-3).10 The ORR with axitinib was 45%, whereas tivozanib fared worse with ORRs of 8–18%.
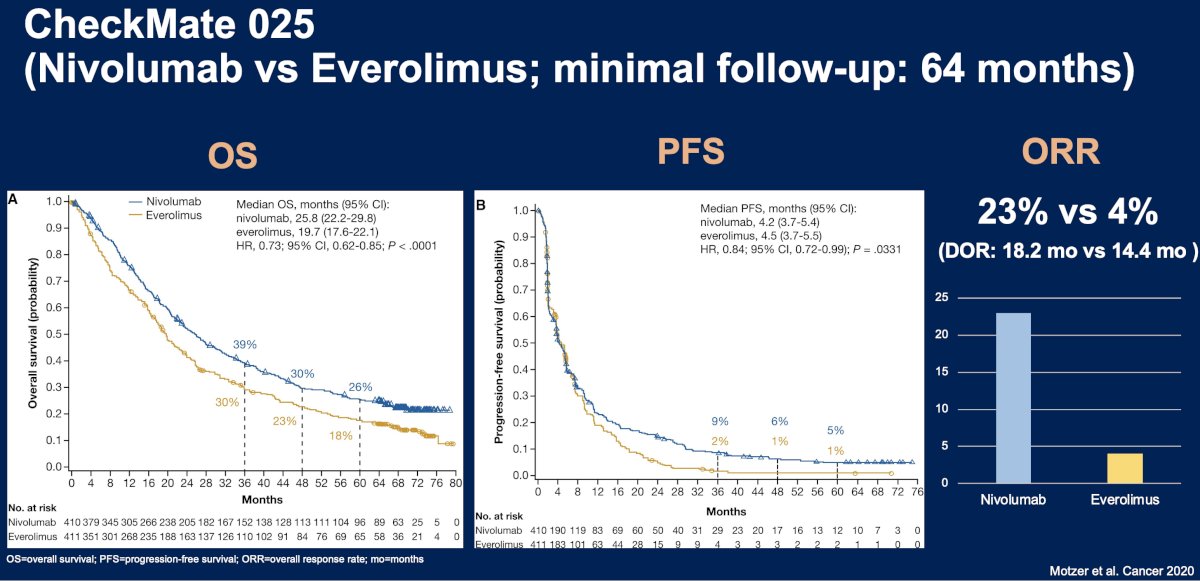
The CheckMate 025 trial is a phase 3 trial that included patients with advanced clear cell RCC previously treated with 1–2 antiangiogenic regimens. Eight hundred twenty-one patients were randomized to nivolumab versus everolimus until progression or unacceptable toxicity. At a median follow-up of 72 months, nivolumab was associated with a superior overall survival benefit compared to everolimus (median, 25.8 versus 19.7 months; HR: 0.73, 95% CI: 0.62–0.85). Median PFS and ORRs both also strongly favored nivolumab.11
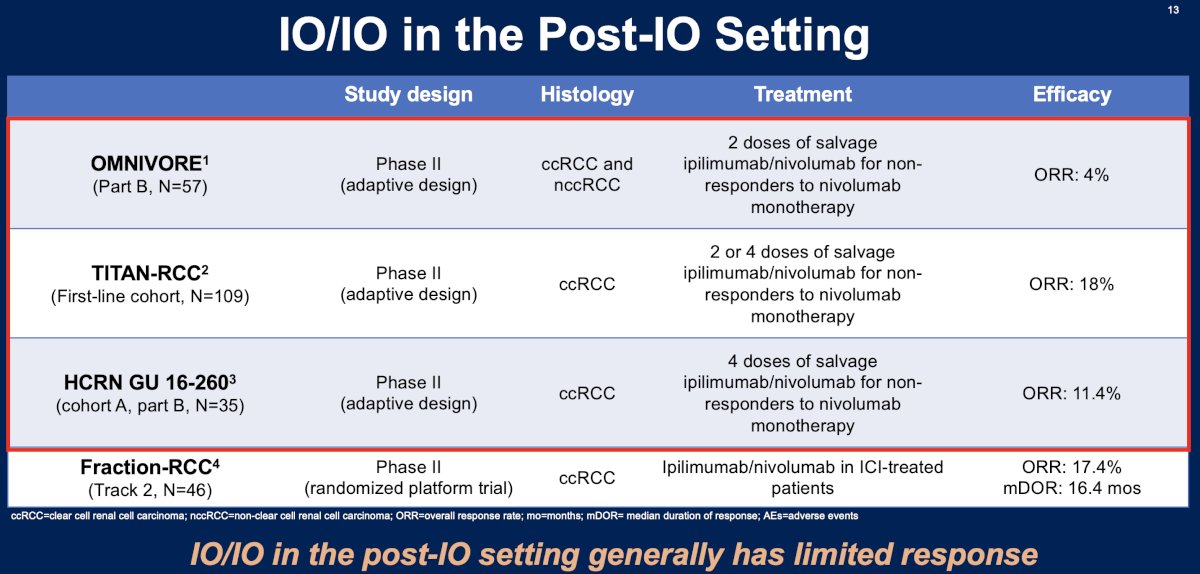
What about IO in the post-IO setting? Overall, trials evaluating IO in the post-IO setting have generally demonstrated limited responses, as summarized in the table below.
What about IO + TKI combination in the post-IO setting? The combination of pembrolizumab + lenvatinib was evaluated in the KEYNOTE-146 trial and fared better compared to the IO agents in this setting with an ORR of 56% and a median duration of response of 12.5 months.12 Cabozantinib +/- atezolizumab was associated with an ORR of 41%, median PFS of ~10.7 months, and a median OS of >2 years.8 The ongoing TiNivo-2 trial is evaluating tivozanib +/- nivolumab in clear cell RCC patients with disease progression following IO therapy.
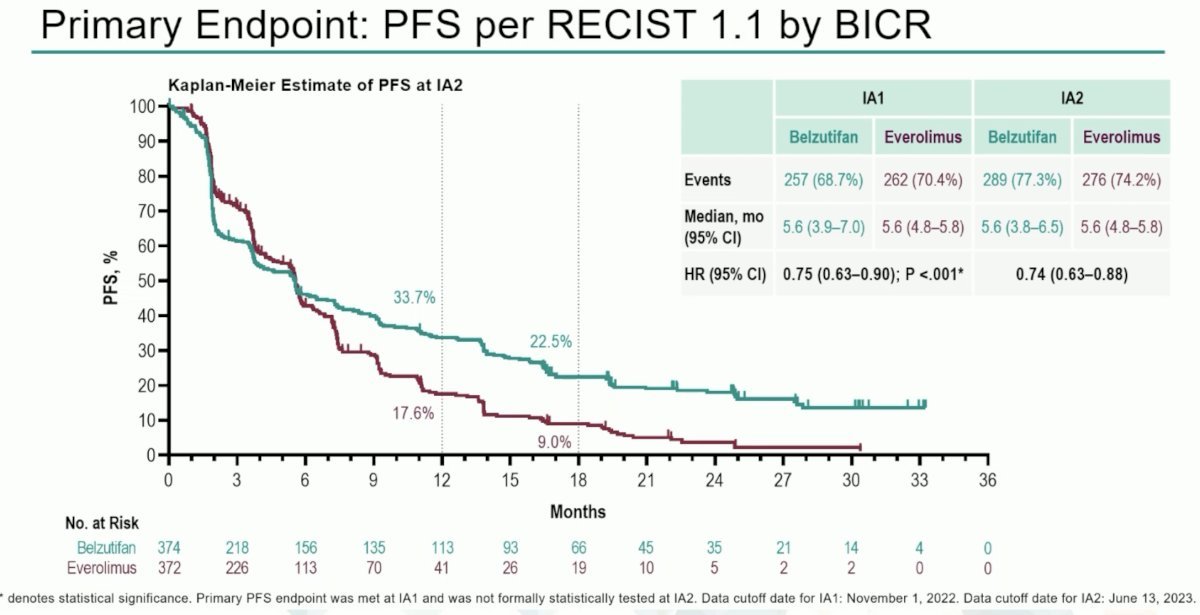
Another agent to be evaluated in this setting is belzutifan. The Hypoxia-Inducible Factor (HIF) pathway is central to the pathophysiology of clear cell RCC and von Hippel-Lindau (VHL) disease. Belzutifan is a first-in-class oral HIF-2a inhibitor that block heterodimerization with HIF-1B and downstream oncogenic pathways. Belzutifan (Welireg®) is approved in the United States for certain VHL disease associated RCC, pancreatic neuroendocrine tumors, and central nervous system hemangioblastomas. Presented at ESMO 2023 by Dr. Laurence Albiges, LITESPARK-005 (NCT04195750) is an open label, randomized phase III study of patients with unresectable, locally advanced, or metastatic ccRCC with evidence of disease progression after 1–3 lines of prior systemic therapy, including ≥1 anti-PD-(L)1 agent and ≥1 VEGFR-TKI. Patients in this study underwent 1:1 randomization to:
- Belzutifan 120 mg orally once daily (n=374)
- Everolimus 10 mg orally once daily (n=372)
At the landmark 18 months analysis, 22.5% of patients remained free of progression with belzutifan, compared to 9% with everolimus (HR: 0.74, 95% CI: 0.63 – 0.88).
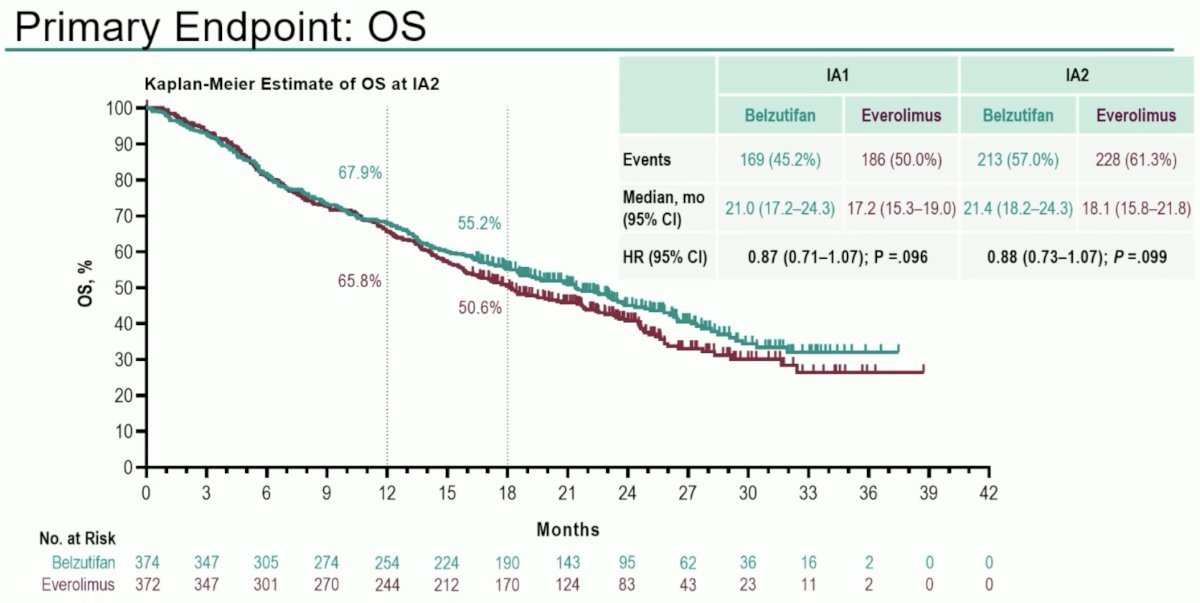
To date, no overall survival benefits have been observed for belzutifan, compared to everolimus, in this study. While there is a signal for OS benefit (HR: 0.88, 95% CI: 0.73 - 1.07, p=0.099), with 18 months OS rates of 55.2% and 50.6% for belzutifan and everolimus, respectively, to date this has not met statistical significance.
An ORR was observed in 22.7% of belzutifan patients, compared to 3.5% for everolimus. A complete response was observed in 3.5% of belzutifan patients, compared to none with everolimus.

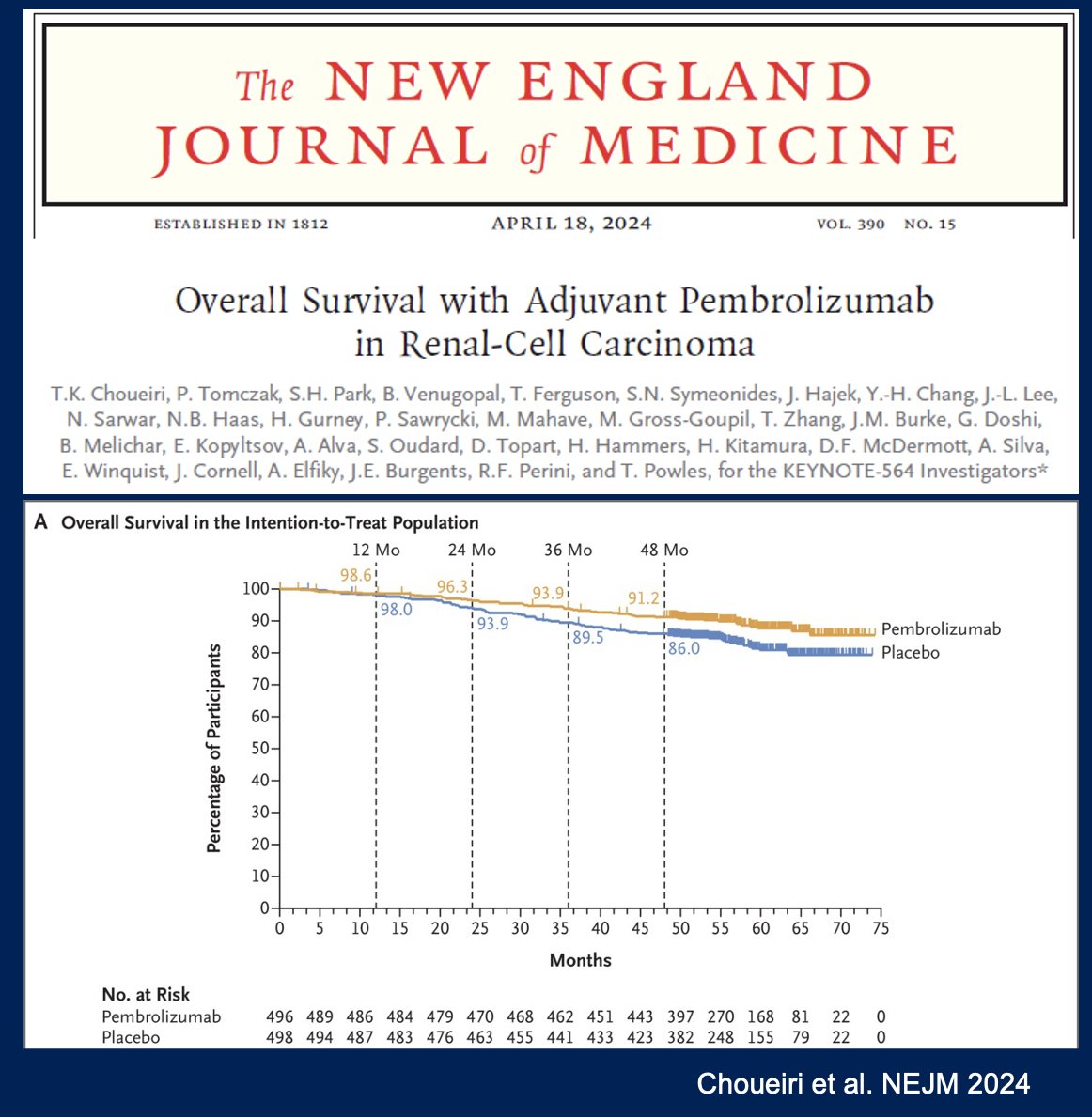
Another emerging paradigm is the treatment of advanced clear cell RCC patients with disease progression following adjuvant pembrolizumab therapy, with KEYNOTE-564 demonstrating an overall survival benefit for pembrolizumab in this setting (48 months: 91.2% versus 86%; HR: 0.62, 95% CI: 0.44–0.87, p=0.005).13
The optimal treatment strategy for these patients will depend on the pattern/timing of recurrence:
- Oligometastatic May consider metastasis-directed therapy with metastasectomy or stereotactic body radiotherapy versus active surveillance in select cases
- Polymetastatic recurrence
- Approach varies depending on timing of recurrence:
- Refractory: During and in the first 3 months after
- Early recurrence: 3–12 months after
- Late recurrence: ≥12 months after
- Approach varies depending on timing of recurrence:

Given the limited evidence to inform post-adjuvant therapy sequencing strategies, it is currently recommended that such patients be considered for clinical trials, where available.
What are some strategies to help more patient while limiting medical/financial toxicities? Early recognition and management of toxicities is critical. These toxicities are class-specific (i.e., IO, TKI, mTOR inhibitor) and need to be identified in a timely fashion.

There are several strategies to recognize and address financial toxicity. This involves:
- Implementing financial toxicity screening:
- NCCN Distress Thermometer
- Practical concerns: work. Housing, finances, insurances, transportation
- COmprehensive Score for financial Toxicity (COST)
- 11-item PROM
- ≤17.5 point (cutoff for financial toxicity)
- NCCN Distress Thermometer
- Providing financial navigation
- Health insurance optimization
- Social support for housing, transportation and other health-related social needs
- Co-payment assistance programs
Ongoing clinical trials investigating financial navigation programs, include:
- SWOG CREDT (NCI04960787)
- CAFÉ study (NCI05018000)
- Addressing Cancer-Related Financial Toxicity in Rural Oncology Care Settings (NCI04931251)
Involving the palliative care team is critical to addressing multiple domains of cancer care.
This is supported by strong evidence demonstrating that involvement of the palliative care in oncologic care improves both quality of life and overall survival.14

Unfortunately, it appears that palliative care remains underutilized in the real-world care of genitourinary malignancies. A retrospective cohort study from the National Cancer Database (NCDB) of stage IV prostate/kidney/bladder cancer patients (n=76,016) demonstrated that palliative therapy was involved in only 20% of kidney cancer cases.15
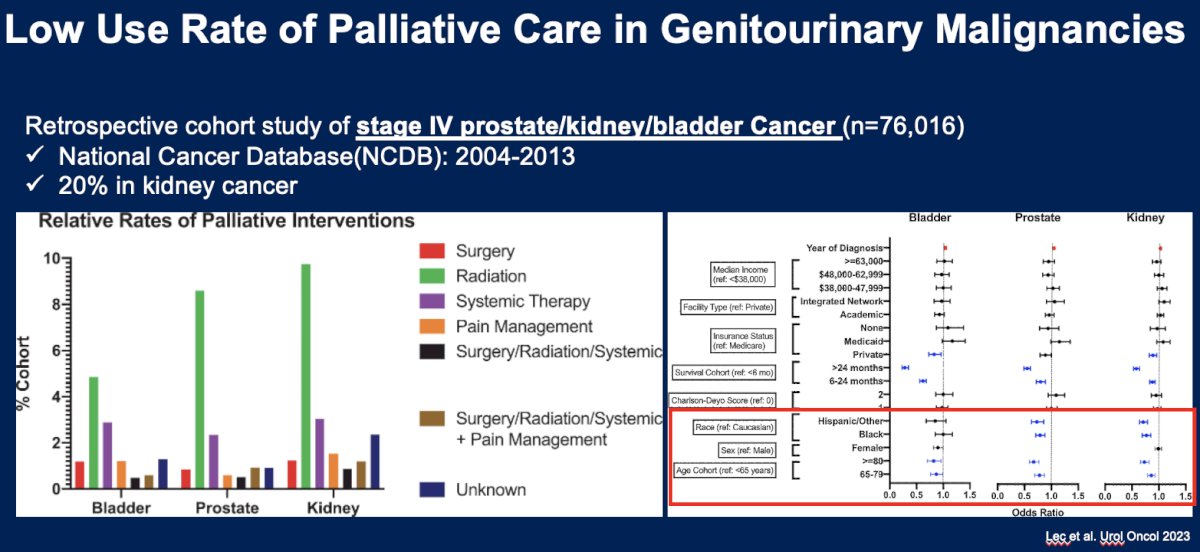
There are a number of barriers to integration of palliative care in this setting, including structural, provider level, and patient/family-related:

Dr. Chen concluded his presentation as follows:
- The current evidence supports VEGF-TKIs and belzutifan after disease progression with prior IO agents.
- IO rechallenging in general does not offer clinical benefit and is associated with increased toxicities.
- Optimal treatment sequencing after adjuvant pembrolizumab in resected high-risk RCC requires future prospective studies.
- Early integration of palliative care improves overall survival and quality of life in cancer care.
Presented by: Yu-Wei Chen, MD, MS, Assistant Clinical Professor, Department of Medicine, University of California, San Diego Medical Center, San Diego, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Shah NJ, Sura SD, Shinde R, et al. Real-world Treatment Patterns and Clinical Outcomes for Metastatic Renal Cell Carcinoma in the Current Treatment Era. Eur Urol Open Sci. 2023;49: 110-8.
- Esterberg E, Iyer S, Nagar SP, et al. Real-World Treatment Patterns and Clinical Outcomes Among Patients With Advanced Renal Cell Carcinoma. Clin Genitourin Cancer. 2024;22(2): 115-25.
- Frazer R, McGrane JM, Challapalli A, et al. Real-world patterns of treatment and response in metastatic renal cell carcinoma: a multicentre UK-wide review with UK Renal Oncology Collaborative (UK ROC). ESMO Real World Data and Digital Oncology. 2024;3:100027.
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807): 1931-9.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N ENgl J Med. 2015;373: 1814-23.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15): 1473-82.
- Tannir NM, Agarwal N, Porta C, et al. Efficacy and Safety of Telaglenastat Plus Cabozantinib vs Placebo Plus Cabozantinib in Patients With Advanced Renal Cell Carcinoma: The CANTATA Randomized Clinical Trial. JAMA Oncol. 2022;8(10): 1411-8.
- Pal SK, Albiges L, Tomczak P, et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2023;402(10397): 185-95.
- Ornstein MC, Pal SK, Wood LS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2019;20(10): 1386-94.
- Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1): 95-104.
- Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18): 4156-67.
- Lee C, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22(7): 946-58.
- Choueiri TK, Tomczak P, Park SH, et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N Engl J Med. 202;390:1359:1371.
- Hoerger M, Wayser GR, Schwing G, et al. Impact of Interdisciplinary Outpatient Specialty Palliative Care on Survival and Quality of Life in Adults With Advanced Cancer: A Meta-Analysis of Randomized Controlled Trials. Ann Behav Med. 2019;53(7): 674-85.
- Lec PM, Tenis AT, Brisbane W, et al. Trends in palliative care interventions among patients with advanced bladder, prostate, or kidney cancer: A retrospective cohort study. Urol Oncol. 2020;38(11): 854.e1-e9.


