(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a kidney and bladder cancers poster session. Dr. Viktor Grünwald presented a follow-up analysis of progression and subsequent therapy patterns from the CLEAR trial of lenvatinib plus pembrolizumab versus sunitinib in advanced clear cell renal cell carcinoma (ccRCC).
In the primary analysis of the phase 3 CLEAR trial, the combination of lenvatinib plus pembrolizumab resulted in statistically significant and clinically meaningful improvements in overall survival compared with sunitinib for patients with advanced renal cell carcinoma.1 These results were further confirmed at the final pre-specified overall survival analysis (Figure 1).2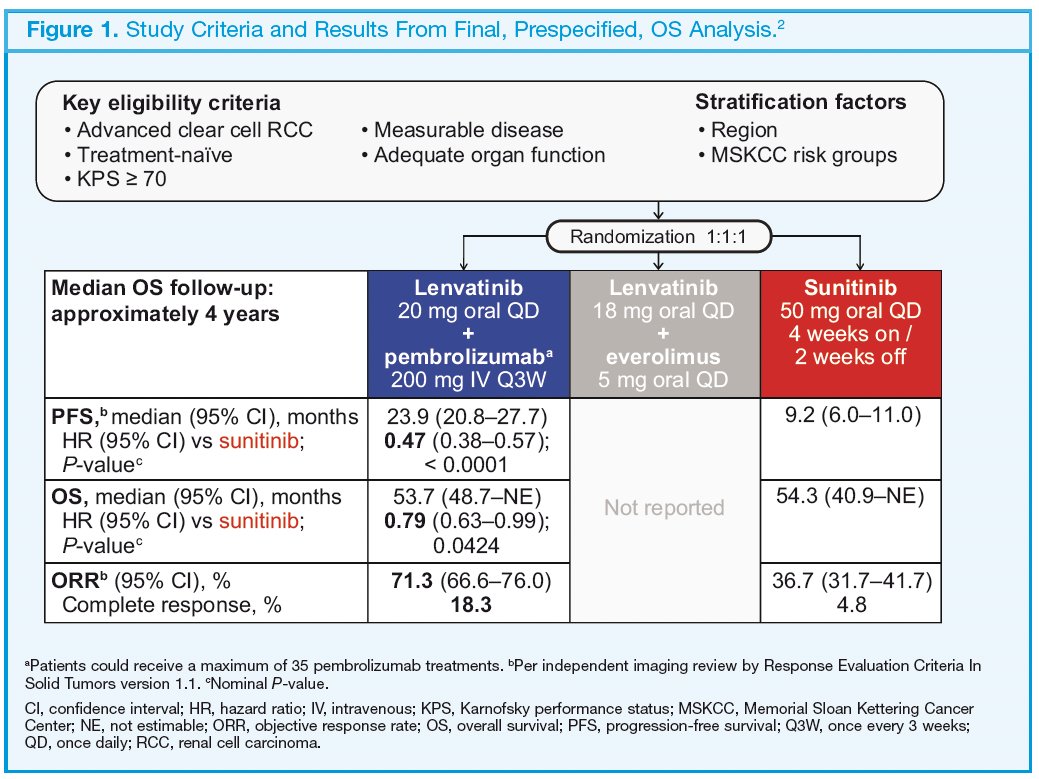
In this report, Dr. Grünwald presented the patterns of progression and subsequent therapy from the CLEAR trial.
The eligibility criteria and dosage information for CLEAR are summarized in the figure above. The objective of this analysis was to evaluate progression patterns within individual organs, with time to progression defined for each organ independently using lesions within each specific organ only, based on independent imaging review per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). Within each organ, progressive disease was defined as a 20% increase in the sum of diameters of target lesion(s) in that organ, progression of non-target lesion(s) in that organ, and/or any new lesion(s) in that organ. Medians were estimated using the Kaplan–Meier method, and 95% confidence intervals were estimated using a generalized Brookmeyer and Crowley method. Hazard ratios (HRs) for lenvatinib plus pembrolizumab versus sunitinib were calculated using Cox regression modeling with treatment as a factor. The Efron method was used for correction of tied events.
In the lenvatinib plus pembrolizumab arm (n = 355), 252 (71%) patients had lung metastases, 162 (46%) patients had lymph node metastases, 80 (23%) patients had bone metastases, and
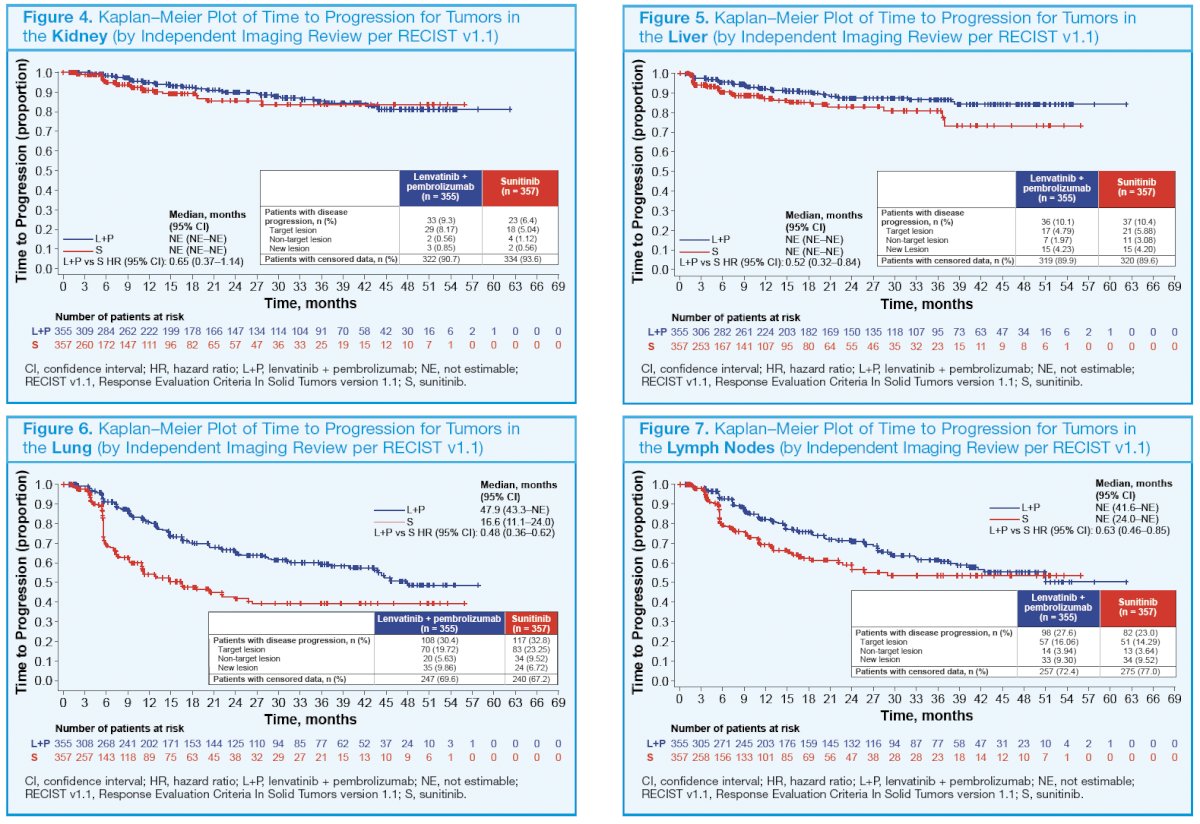
63 (18%) patients had liver metastases at baseline. Correspondingly, in the sunitinib arm (n = 357), 228 (64%) patients had lung metastases, 156 (44%) had lymph node metastases, 89 (25%) patients had bone metastases, and 70 (20%) patients had liver metastases at baseline. The number of patients with a baseline brain metastasis across treatment arms was very low (≤ 10). The HRs for time to disease progression favored lenvatinib plus pembrolizumab versus sunitinib treatment across tumors in different organs (bone, central nervous system, kidney, liver, lung, and lymph nodes; Figure 2–7).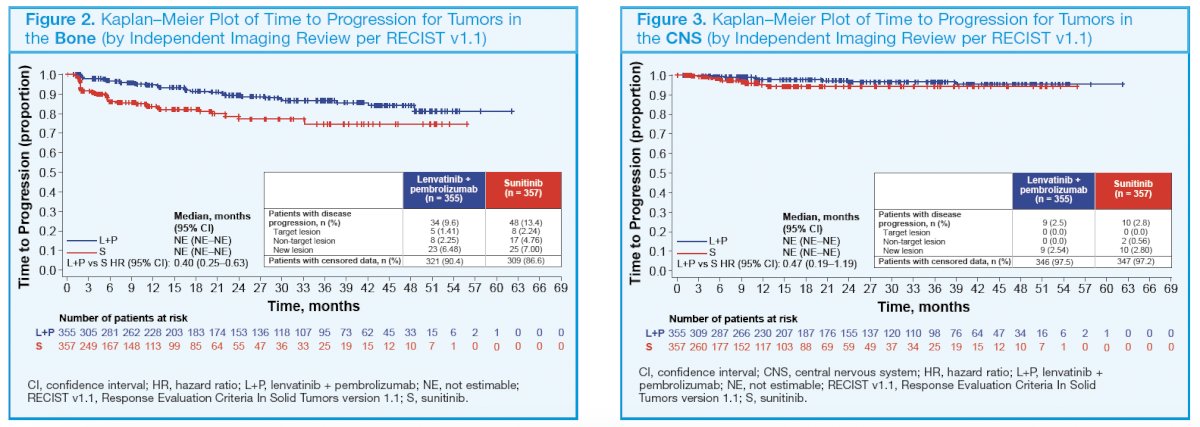
At the time of overall disease progression, the median decreases in percent changes in sums of diameters of target lesions were greater with lenvatinib plus pembrolizumab versus sunitinib treatment (−48.1% vs −17.4%; Figure 8).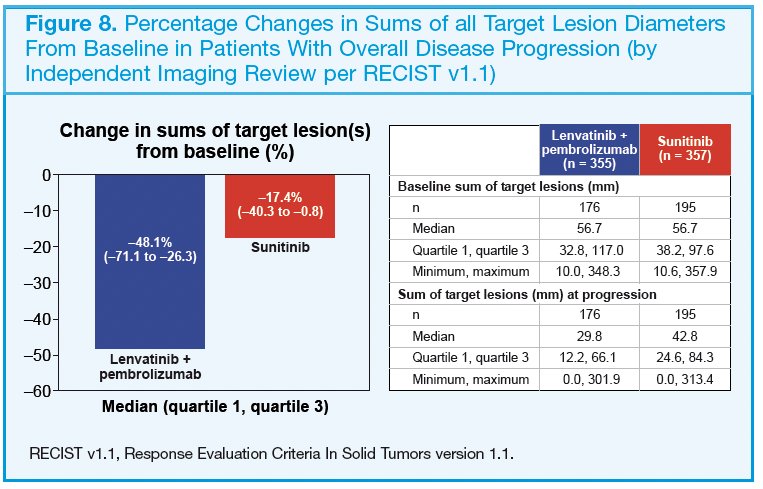
In the lenvatinib plus pembrolizumab arm, 181 patients received subsequent anti-cancer regimens during survival follow up:
- 43 patients received axitinib
- 101 received cabozantinib
- 42 received nivolumab
In the sunitinib arm, 246 patients received subsequent anticancer regimens:
- 47 patients received axitinib
- 107 received cabozantinib
- 175 received nivolumab
Patients in the lenvatinib plus pembrolizumab arm continued with second-line axitinib or cabozantinib treatment longer than those in the sunitinib arm (Table 1).
Dr. Grünwald concluded as follows:
- In all assessed organs, the time to tumor progression was prolonged with lenvatinib plus pembrolizumab versus sunitinib treatment
- At the time of overall disease progression, the median tumor burden of target lesions was lower with lenvatinib plus pembrolizumab versus sunitinib treatment.
- Patients in the lenvatinib plus pembrolizumab arm remained on second line axitinib or cabozantinib treatment longer compared to patients in the sunitinib arm.
- Together, these results continue to support lenvatinib plus pembrolizumab treatment as a standard-of-care first-line therapy in patients with advanced ccRCC.
Presented by: Viktor Grünwald, MD, PhD, Professor, Interdisciplinary Genitourinary Oncology, University Hospital Essen, Essen, Germany
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021 Apr 8;384(14):1289-1300.
- Motzer RJ, Porta C, Eto M, et al. Lenvatinib Plus Pembrolizumab Versus Sunitinib in First-Line Treatment of Advanced Renal Cell Carcinoma: Final Prespecified Overall Survival Analysis of CLEAR, a Phase III Study. J Clin Oncol. 2024;42(11): 1222-8.


