(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a kidney and bladder cancers poster session. Dr. Peter Goebell presented a real-world comparison of checkpoint inhibitors (CPIs) versus tyrosine kinase inhibitors (TKIs) for the first line treatment of advanced renal cell carcinoma (RCC) patients using data from the CARAT German research platform.
CPIs in combination with TKIs or with another CPI have expanded the therapeutic options for advanced or metastatic RCC beyond TKI monotherapy. However, head-to-head phase Ill randomized trials comparing first-line CPI + CPI versus CPI+ TKI are lacking. In this study, Dr. Goebell and colleagues compared the effectiveness of these strategies by emulating a hypothetical randomized trial using a large, prospectively collected real-world dataset from CARAT.
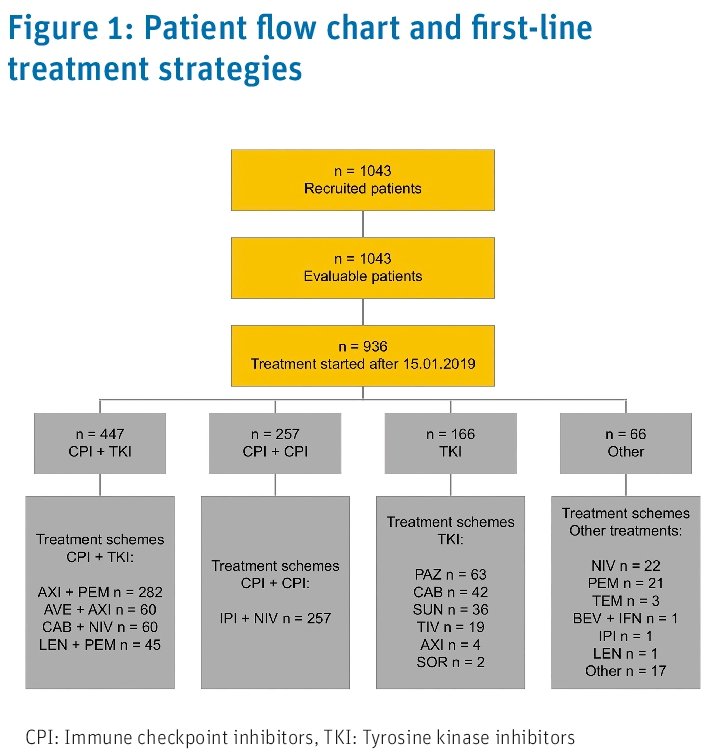
The RCC Research Platform, CARAT is an ongoing, prospective, observational, longitudinal, multicenter clinical registry collecting data on molecular testing, patient, and tumor characteristics, (sequential) treatments, and course of disease in patients with mRCC in Germany (NCT03374267). Patients are followed until death or for a maximum of three years. By April 2024, 148 sites (hospitals and office-based practices) recruited about 1,150 patients at start of the first line treatment. For the present work, those patients who started first line therapy after January 15th, 2019 were analyzed (data cut-off date: December 31st, 2023).
The primary and secondary endpoints were progression-free survival and overall survival, respectively. These were estimated using the Kaplan Meier method. The outcomes were also calculated stratified by patients’ prognostic risk according to the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model.
To emulate a hypothetical randomized trial, inverse probability of treatment weighting using stabilized propensity score weights was used to compare progression free survival and overall survival between first line CPI + TKI, CPI + CPI, and TKI. Inverse probability of treatment weighting was performed for the following variables:
- Age
- Sex
- IMDC risk group
- Histology
- Any comorbidity
- Charlson comorbidity index
- Metastatic stage
- Type of metastasis
- Number of metastatic sites
Treatment strategies were compared using the hazard ratio with 95% confidence intervals based on Cox’s proportional hazards model. Patient reported outcomes were assessed using the validated health related quality of life questionnaire, NCCN-FACT FSKI-19. Patients were asked to fill in questionnaires at the time of recruitment at baseline and every three months thereafter for up to 24 months. Time to deterioration was implemented as a measure of longitudinal health-related quality of life and defined as the time from the start of first line treatment to the time of first clinically relevant deterioration of health-related quality of life or death. Patient reported outcomes will be available in future reports.
At the data cut off for this analysis, 1,043 patients had been recruited including 936 patients who started first line treatment after January 15th, 2019. 447 patients (48%) received CPI+TKI, 257 (27%) CPI + CPI, 166 (18%) TKI monotherapy, and 66 (7%) another regimen as first-line treatment.
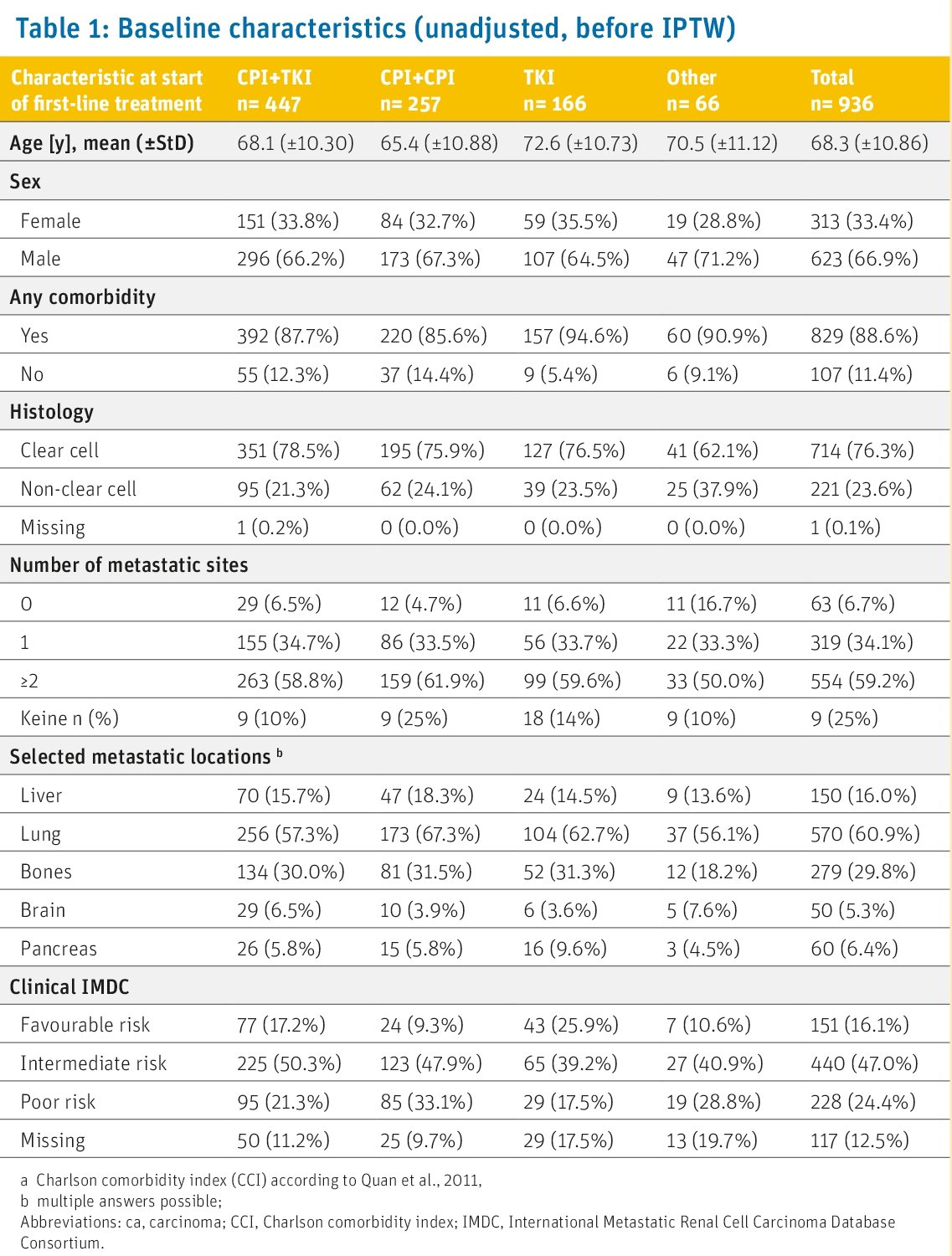
Baseline characteristics of patients before inverse probability of treatment weighting are shown in Table 1 below. 67% of patients (n=693) were male; the majority (89% n=829) presented with comorbidities. Patients receiving CPI + CPI were younger (65 years) and showed less favorable IMDC risk (9%) than patients treated with CPI + TKI or TKI alone.
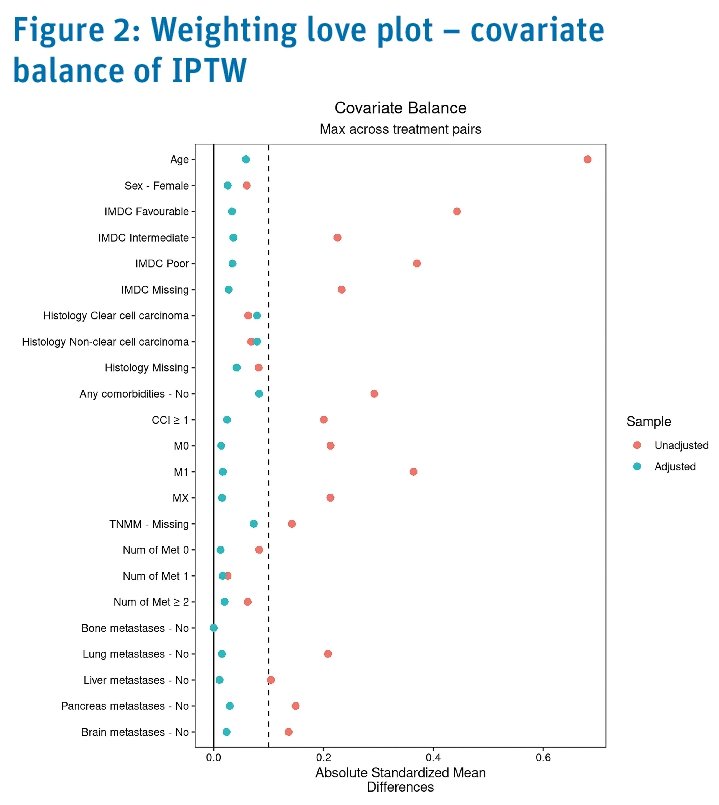
After inverse probability of treatment weighting, the treatment groups were comparable for all variables listed above.
For 340 patients (37%), first-line treatment was ongoing at the time of data cut-off, 332 patients (36%) had received second-line treatment, while 210 patients (22%) had died prior to second-line, and 45 patients (5%) were lost to follow-up.
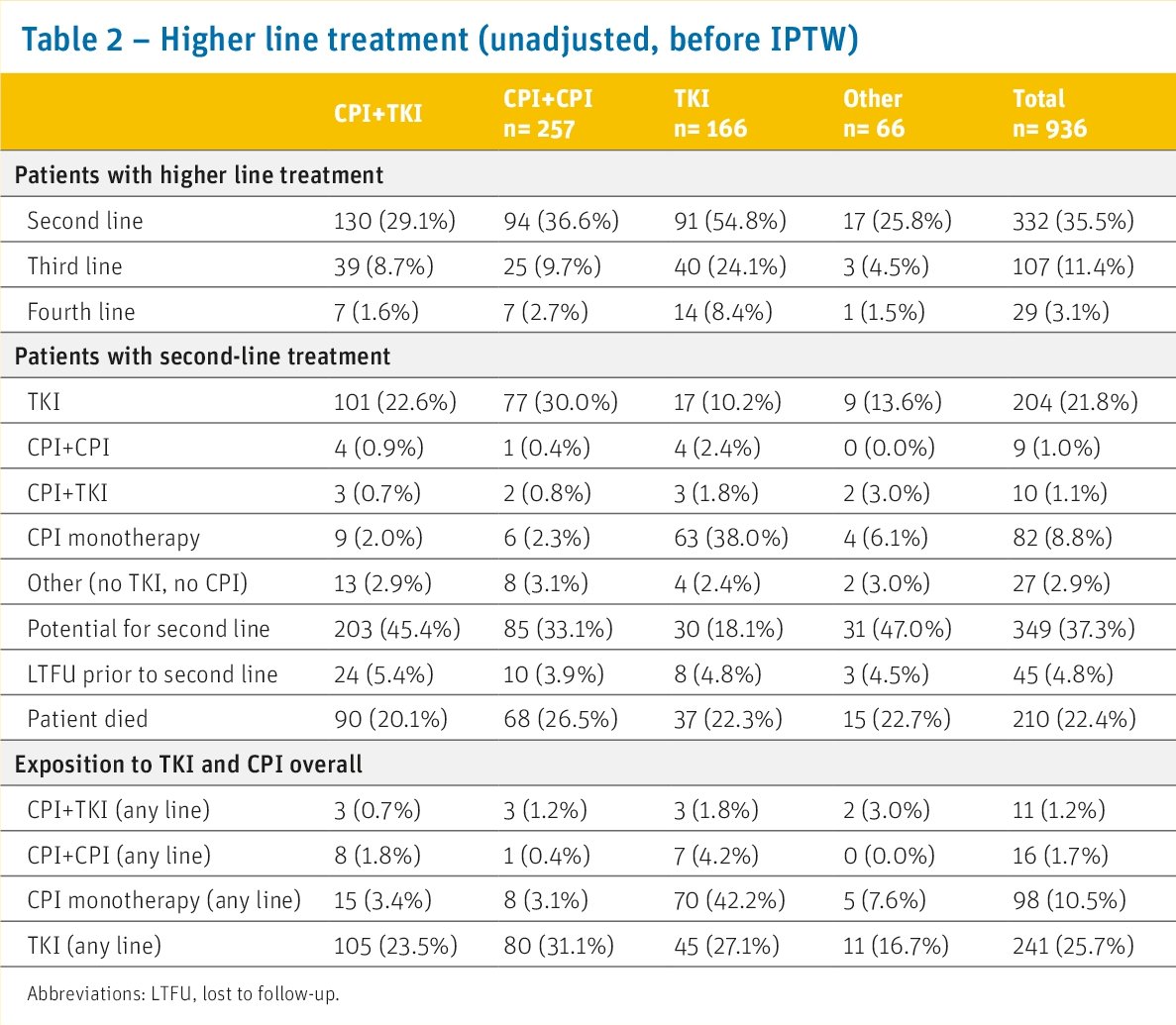
How was this population different from those from pivotal trials in this space? In this data set, patients were older (68 versus 61–64 years), were less frequently male (67% versus 71–77%), less frequently of favorable (11% versus 20–31%) and more frequently of poor (29% versus 9–20%) prognostic risk according to IMDC than in the pivotal trials comparing CPI + TKI or CPI + CPI strategies against sunitinib. Of all first line TKI monotherapy patients receiving a second line treatment (n=91, 55%), the majority (n=70, 77%) were treated with a CPI in the second-line setting, including 63 patients (70%) with CPI monotherapy. The proportion is slightly higher than in most pivotal trials comparing CPI + TKI or CPI + CPI strategies against sunitinib (48% versus 34–40%).
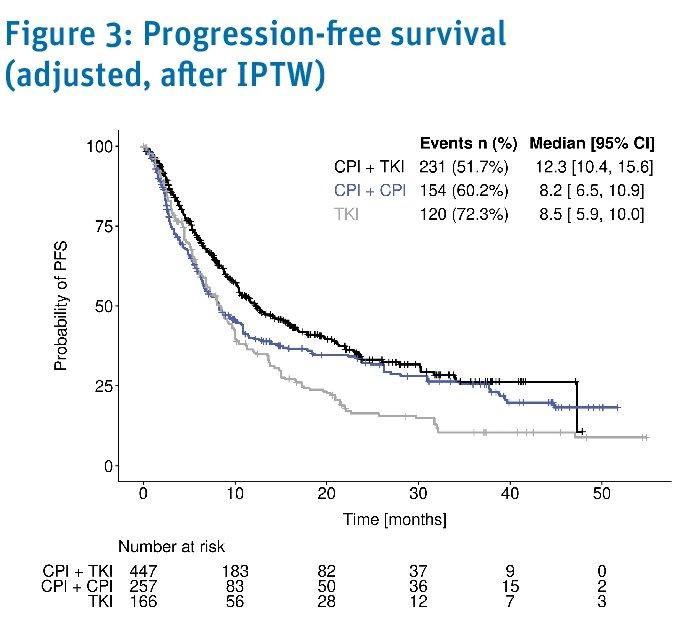
Progression or death after 1st line treatment was experienced by:
- CPI + TKI: 52%
- CPI + CPI: 60%
- TKI monotherapy: 72%
The inverse probability treatment weighting-adjusted median progression free survivals were:
- TKI + CPI: 12.3 months
- CPI + CPI: 9.2 months
- TKI: 8.5 months
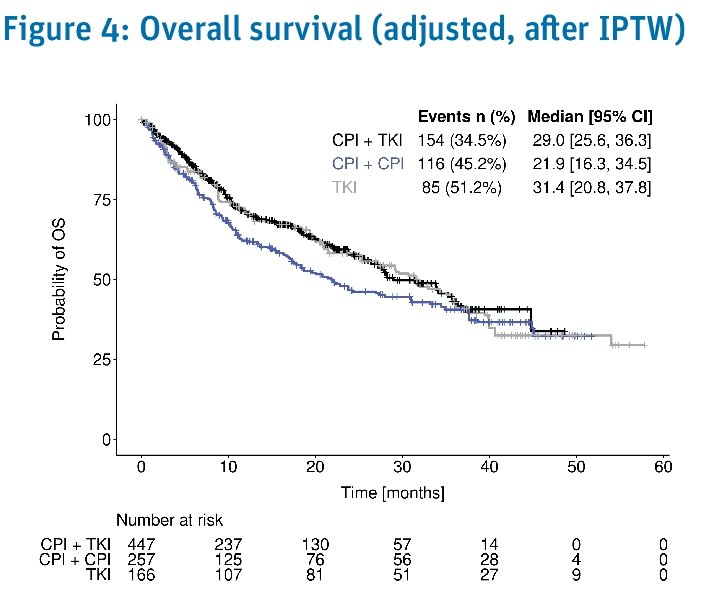
The inverse probability treatment weighting-adjusted median overall survivals were:
- TKI + CPI: 29 months
- CPI + CPI: 21.9 months
- TKI: 31.4 months
When stratified by patients’ prognostic risk according to the IMDC, there was no difference in outcomes between the three strategies.
Limitations to this study include:
- Emulated randomized trials are by design limited by the lack of randomization, and the number and quality of potentially confounding variables are essential in guiding interpretability. It cannot be excluded that unknown confounders may have affected the results.
- Crossing survival curves indicate that the hazard ratios should be interpreted with care since the proportional hazards assumption is violated. Restricted mean survival times will be presented in later reports.
Dr. Goebell concluded as follows:
- The results of this analysis using a large real-world dataset from the CARAT registry indicate no statistically significant differences in progression-free and overall survivals of first line CPI + CPI compared to CPI + TKI when adjusted for a wide range of potential confounding variables.
- However, there is a trend towards a better survival with first line CPI + TKIm compared to CPI + CPI.
- Differences in the choice of first line TKI and subsequent treatment might explain differences in median progression free and overall survival in the present analysis, compared to recently published phase three trials.
- While head-to-head phase three randomized clinical trials on these first line strategies in metastatic RCC are lacking, the emulation of a hypothetical target trial using real-world data may help fill this knowledge gap.
- Further analysis in other real-world cohorts or preferentially randomized clinical trials is warranted to confirm these findings.
Presented by: Peter J. Goebell, MD, PhD, Division of Urology, University Hospital Erlangen, Erlangen, Germany
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


