(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a kidney and bladder cancers poster session. Dr. Talal Zarif presented the results of an international, multi-institutional analysis of first-line systemic therapy following adjuvant immunotherapy in renal cell carcinoma (RCC) patients.
KEYNOTE-564 demonstrated an overall survival benefit for the adjuvant treatment of RCC patients at high risk of recurrence following a radical nephrectomy with pembrolizumab (48 months overall survival: 91.2% versus 86%; HR: 0.62, 95% CI: 0.44–0.87, p=0.005).1 A proportion of RCC patients receiving adjuvant immunotherapy will experience subsequent disease recurrence. The objective of this study was to evaluate the clinical outcomes of patients receiving first line systemic therapy following RCC recurrence on/after adjuvant immunotherapy.
This was a retrospective analysis of RCC patients with disease recurrence during/following adjuvant immunotherapy who received 1st line systemic therapy at 29 institutions. Progression-free and overall survivals were estimated using the Kaplan-Meier method. The investigators also assessed treatment-related adverse events leading to treatment discontinuation, dose reduction, or corticosteroid use.
The baseline patient characteristics are summarized below. The median patient age was 58 years. 40% of patients were from Europe and almost 50% were from the United States or Canada. Two-thirds had M0 intermediate-to-high risk disease at baseline. 17% of patients had non-clear cell histology, and 16% had sarcomatoid features.
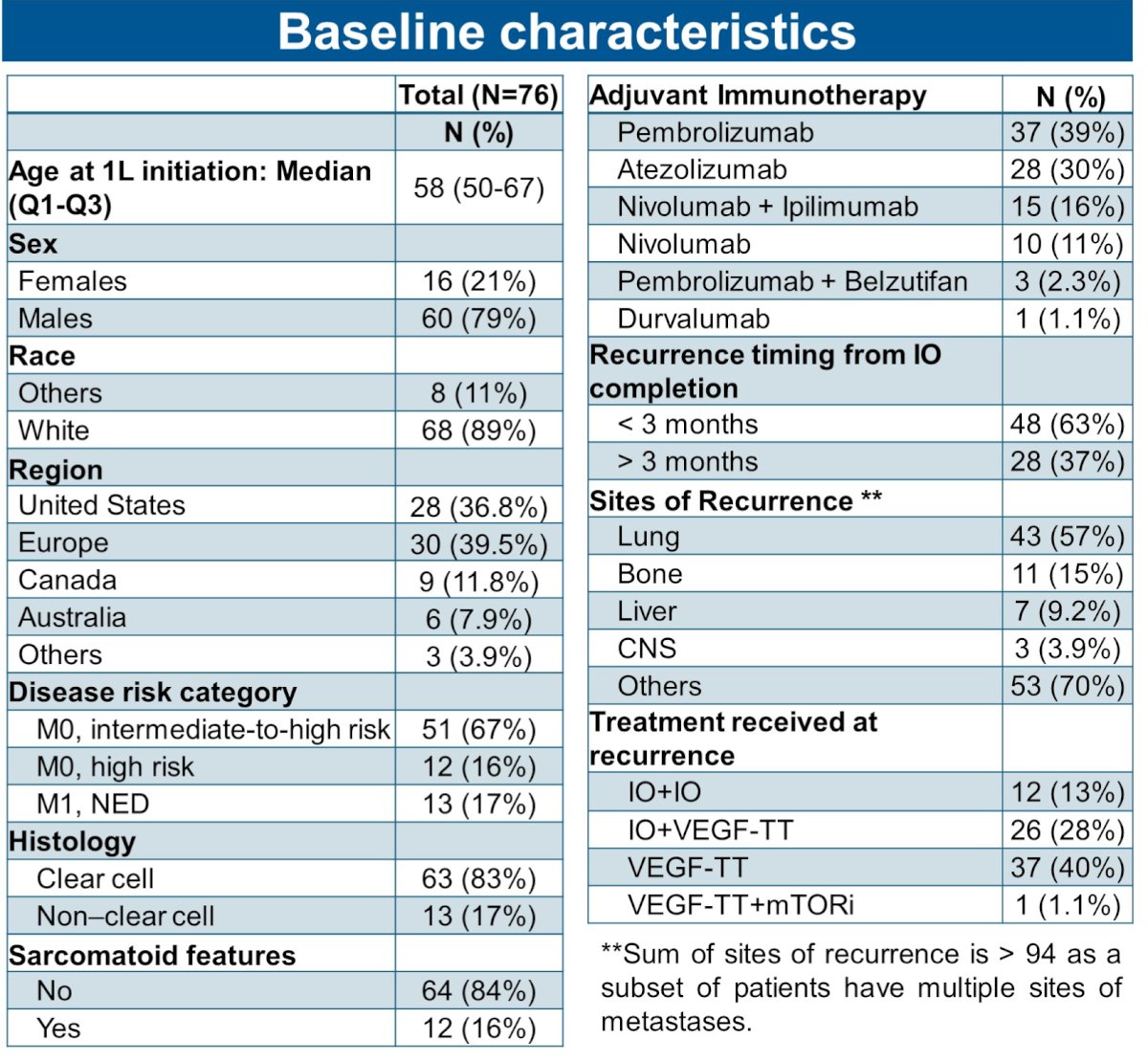
The choice of adjuvant immunotherapy was pembrolizumab in 40% of patients, followed by atezolizumab (30%), nivolumab + ipilimumab (16%), and nivolumab monotherapy (11%). The most common reasons for adjuvant immunotherapy cessation were:
- Treatment completion (n=65, 45%)
- Disease recurrence (n=36, 25%)
- Treatment toxicity (n=34, 24%)
The median follow-up from systemic therapy initiation was 16.7 months. Disease recurrence was observed in 92 (64%) patients. Following tumor recurrence, 69/92 (75%) patients received systemic therapy; the remaining 25% underwent surgery or radiotherapy. The median progression-free survival was 16 months, and the 18 months overall survival was 86%.
Following disease recurrence, most patients received:
- VEGF inhibitors: 46%
- IO + VEGF inhibitors: 30%
- IO monotherapy or IO + IO: 17%
The objective response rates were comparable among these three subsequent treatment groups:
- VEGF inhibitors: 35%
- IO + VEGF inhibitors: 45%
- IO monotherapy or IO + IO: 42%
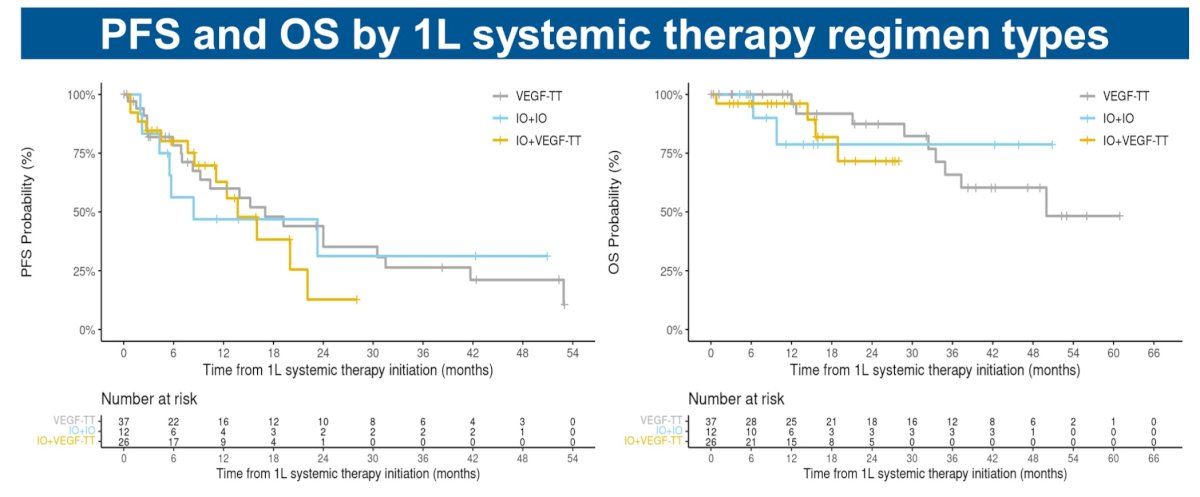
There were no significant differences in progression-free and overall survivals by 1st line systemic therapy regimen type administered:
In the 23 patients who received adjuvant pembrolizumab monotherapy and subsequent systemic therapy, the ORR was 44% (4/9) for those recurring after pembrolizumab discontinuation and 29% (4/14) for those recurring prior to pembrolizumab discontinuation.
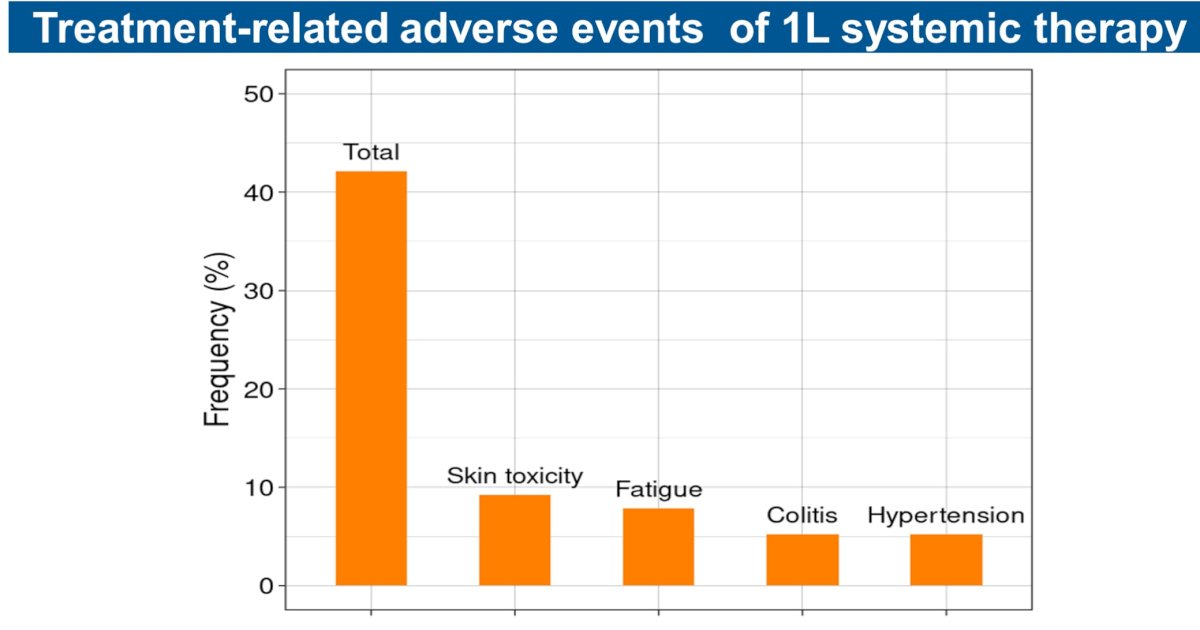
Two of eight patients who discontinued adjuvant immunotherapy due to toxicity were re-challenged with subsequent immunotherapy and had stable disease, with one of them developing an immune-related adverse event. Treatment-related adverse events occurred in 28/60 (41%) patients treated with subsequent systemic therapy.

Dr. El Zarif concluded that patients with recurrent RCC following adjuvant immunotherapy benefit from subsequent systemic therapies, including VEGF inhibitor and immunotherapy regimens.
Presented by: Talal El Zarif, MD, Resident Physician, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Related content: Understanding KEYNOTE-564: Pembrolizumab’s Role and Post-Adjuvant Therapy for RCC Patients - Karl Semaan & Talal El Zarif
References:


