(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a kidney and bladder cancers poster session. Dr. Micah Ostrowski presented an analysis of the comparative effectiveness of first-line ipilimumab plus nivolumab versus immune checkpoint inhibitors plus tyrosine kinase inhibitors in patients with intermediate or poor risk metastatic clear cell renal cell carcinoma (ccRCC).
Immuno-oncology (IO)-based therapies, either with IO-IO (ipilimumab + nivolumab) or IO + tyrosine kinase inhibitors (TKIs), are currently recommended in the 1st line treatment setting for intermediate and poor-risk metastatic ccRCC patients. With no head-to-head prospective trials directly comparing the effectiveness of IO + IO to IO + TKI, the comparative effectiveness of these two combination regimens remain unclear. In this study, the investigators sought to compare the survival outcomes of patients receiving 1st line ipilimumab + nivolumab to IO + TKIs in a real-world setting.
This was a retrospective study using the nationwide Flatiron Health electronic health record database. Patients were included from approximately 280 cancer clinics. Inclusion criteria were as follows:
- Metastatic RCC with clear cell histology
- IMDC intermediate-poor risk disease
- Received 1st line ipilimumab + nivolumab or IO + TKI (i.e. axitinib + pembrolizumab, cabozantinib + nivolumab, lenvatinib + pembrolizumab or axitinib + avelumab) between November 2016 and January 2023
The primary endpoints were overall survival and time-to-next therapy. These were summarized using Kaplan-Meier survival estimates with 95% confidence intervals and compared in the context of propensity score matching weighted analysis and Cox proportional hazard modeling. The propensity score model included the baseline covariates of age, race, smoking status, practice type, insurance, year of therapy, IMDC risk factors (all six), and missingness of covariates. All analyses were performed using R version 4.2.3.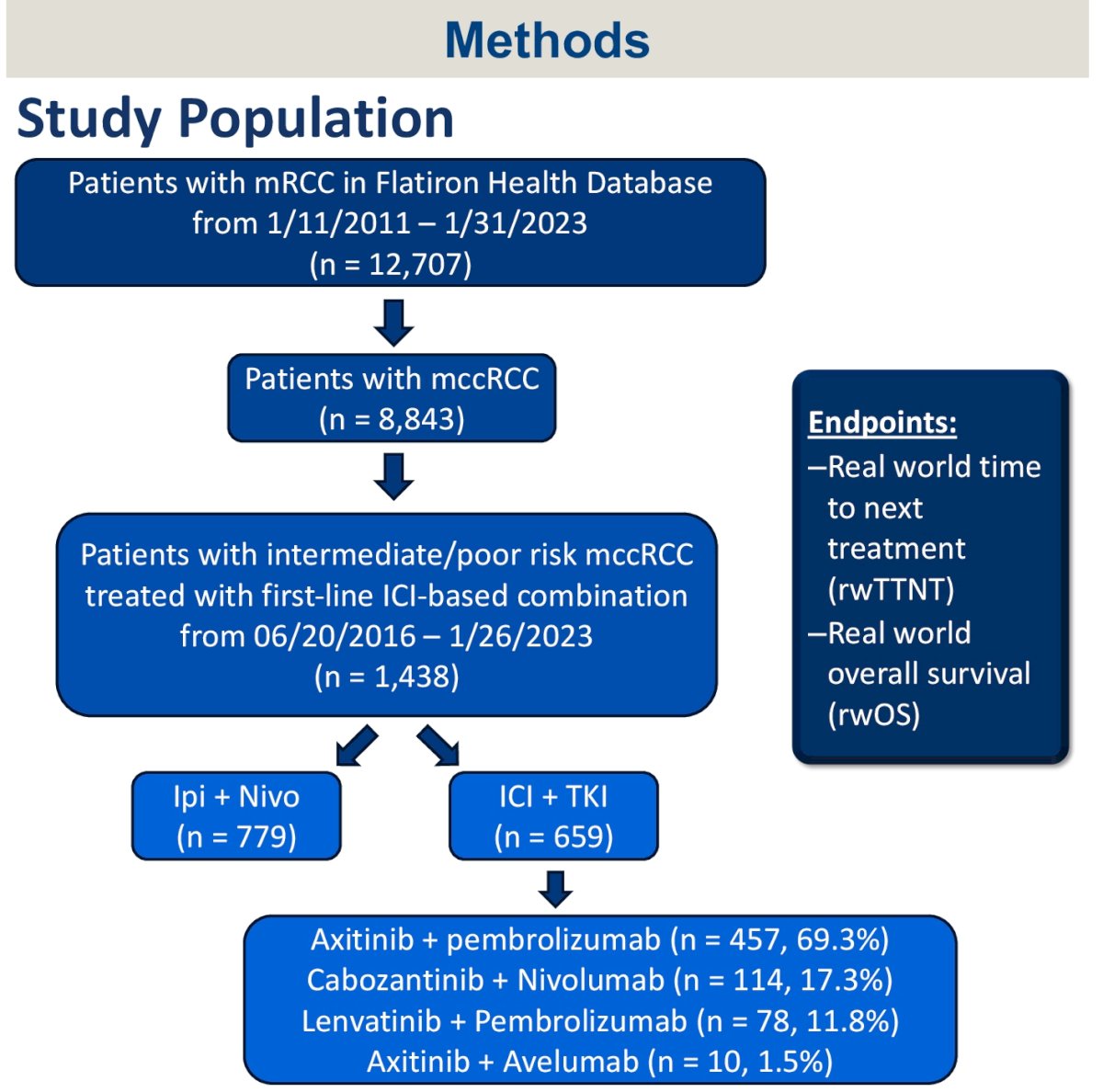
Of 12,707 patients with metastatic RCC in the dataset, 1,438 met the eligibility criteria and were included. The baseline patient characteristics are summarized below: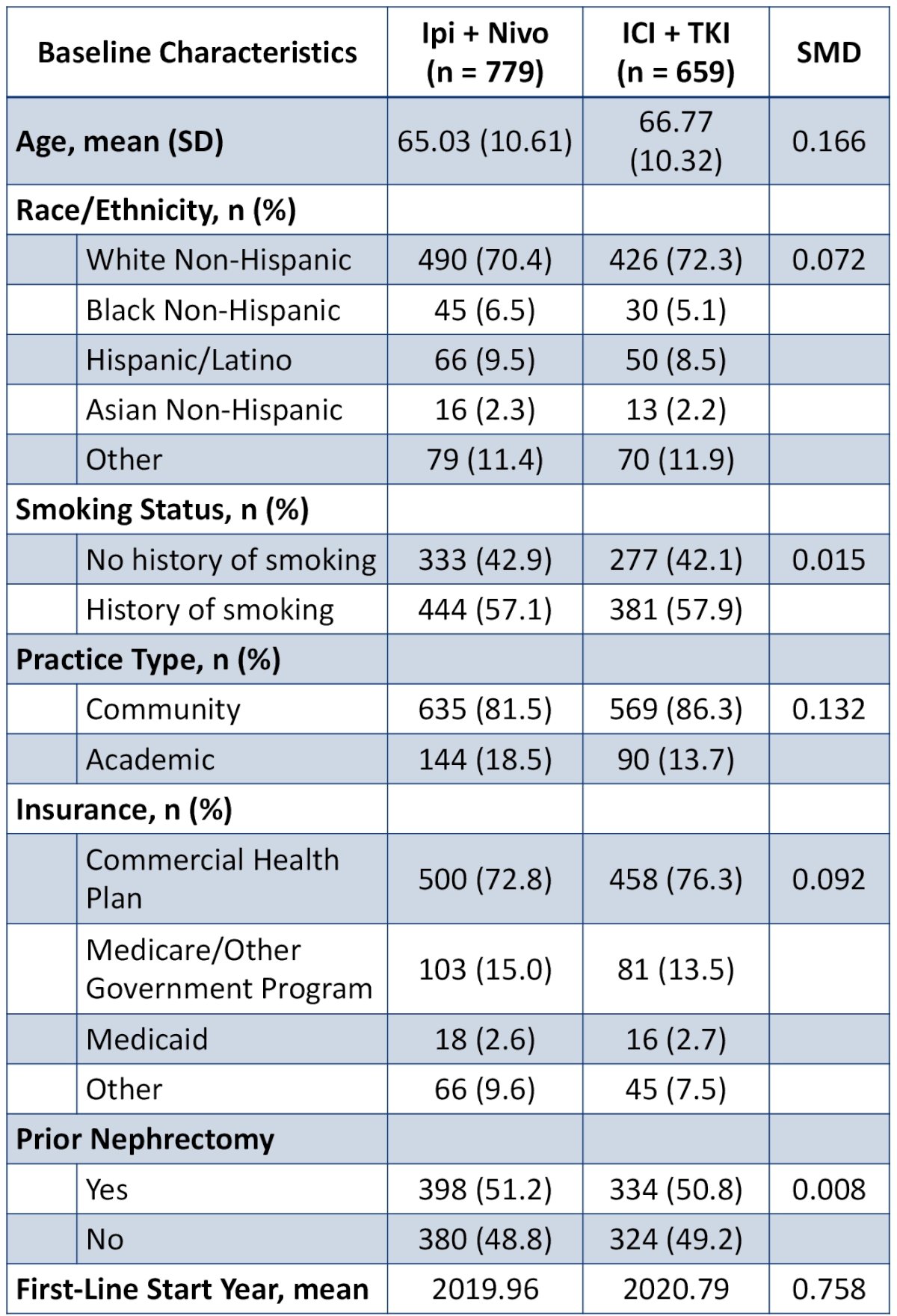
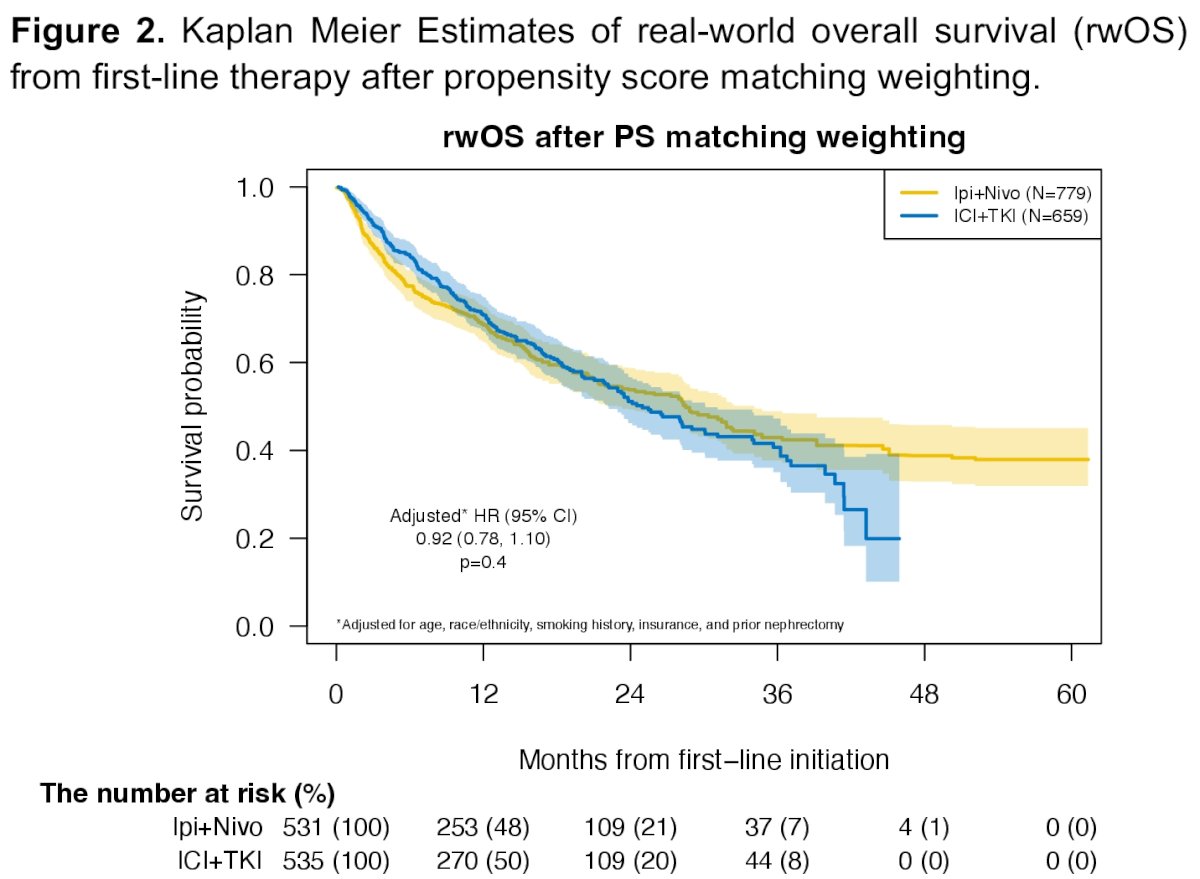
The median overall survival for ipilimumab + nivolumab was 30 months, compared to 34 months for IO + TKIs (HR: 1.02, 95% CI: 0.84 – 1.24, p=0.81). The median time-to-next therapy was 9.1 months for ipilimumab + nivolumab versus 15 months for IO + TKIs (HR: 1.29, 95% CI: 1.09 – 1.51, p=0.002).
After propensity score matching, no significant difference in overall survival was noted between both groups (HR: 1.01, 95% CI: 0.83 – 1.24, p=0.89). However, time-to-next therapy was significantly shorter with ipilimumab + nivolumab, compared to IO + TKI (HR: 1.24, 95% CI: 1.05 – 1.47, p=0.011).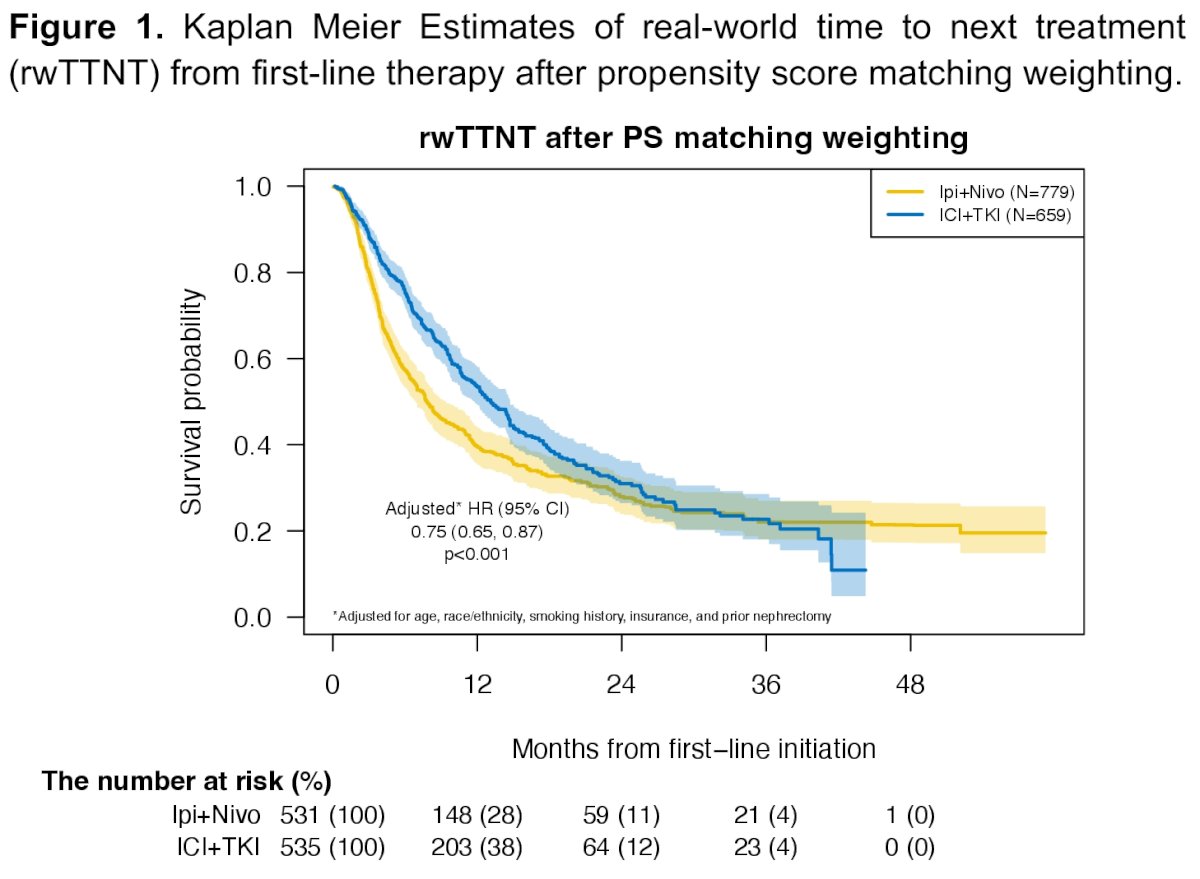
Dr. Ostrowski concluded as follows:
- Patients with metastatic ccRCC treated with first-line IO + TKI have superior time-to-next therapy and similar overall survival, compared to those treated with first-line ipilimumab + nivolumab.
- In the absence of head-to-head clinical trials comparing first-line IO-based combinations or biomarkers, these real-world findings offer important guidance to clinicians for choosing between TKI-containing regimens or ipilimumab with nivolumab as frontline treatment.
Presented by: Micah Ostrowski, MD, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024


