(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a kidney and bladder cancers trials-in-progress poster session. Dr. Sumanta Pal presented the results of the randomized phase III STELLAR-304 trial evaluating the combination of zanzalintinib (XL092) plus nivolumab in patients with non-clear cell renal cell carcinoma (nccRCC).
nccRCC accounts for ~25% of all renal tumors and is a heterogenous group, including papillary, chromophobe, collecting duct, medullary and translocation-associated, and unclassified histology renal tumors, with each showing a distinct presentation, clinical course, and treatment response. Given the rarity and heterogeneity of the disease, management of nccRCC is primarily based on results from phase II trials, subgroup analyses of RCC trials, meta-analyses, and treatments approved for clear cell RCC.
The tyrosine kinase inhibitor (TKI) sunitinib is a commonly used therapy based on the results of two randomized phase 2 studies that showed modest improvements in progression-free survival (PFS) versus everolimus for patients with nccRCC.1,2 Single-agent nivolumab (an anti-PD-1 immune checkpoint inhibitor, ICI) has demonstrated antitumor activity in patients with nccRCC; when combined with cabozantinib, a multi-targeted TKI, with promising clinical activity observed in patients with papillary, unclassified, or translocation-associated nccRCC.3,4
To date, no treatment for nccRCC has demonstrated an overall survival (OS) benefit versus sunitinib, highlighting the need for new treatment strategies. Zanzalintinib (XL092) is a novel, oral, multi-targeted TKI of vascular endothelial growth factor receptor (VEGFR), MET, and the TAM family of kinases TYRO3, AXL, and MER.

VEGFR, MET, and the TAM kinases are involved in tumor cell proliferation, neovascularization, and immunosuppression within the tumor microenvironment. Concomitant inhibition of VEGFR, MET, and the TAM kinases may prevent resistance to VEGFR inhibition. VEGFR, MET, and the TAM kinases are implicated in suppression of antitumor immunity. Inhibition of these kinases may promote an immune-permissive environment and enhance response to ICIs. Based on preclinical analyses, the combination of zanzalintinib and ICI exhibits greater tumor growth inhibition than single-agent therapy. In a phase 1 study, zanzalintinib plus an ICI showed promising antitumor activity with a manageable safety profile in patients with advanced solid tumors including RCC.5
STELLAR-304 (NCT05678673) is a global, open-label, randomized, phase III trial that will enroll approximately 291 patients aged ≥18 years with histologically confirmed unresectable, locally advanced, or metastatic nccRCC who have not received prior systemic anticancer therapy for advanced disease. Eligible patients will be randomized 2:1 to receive oral zanzalintinib plus intravenous nivolumab versus oral sunitinib.
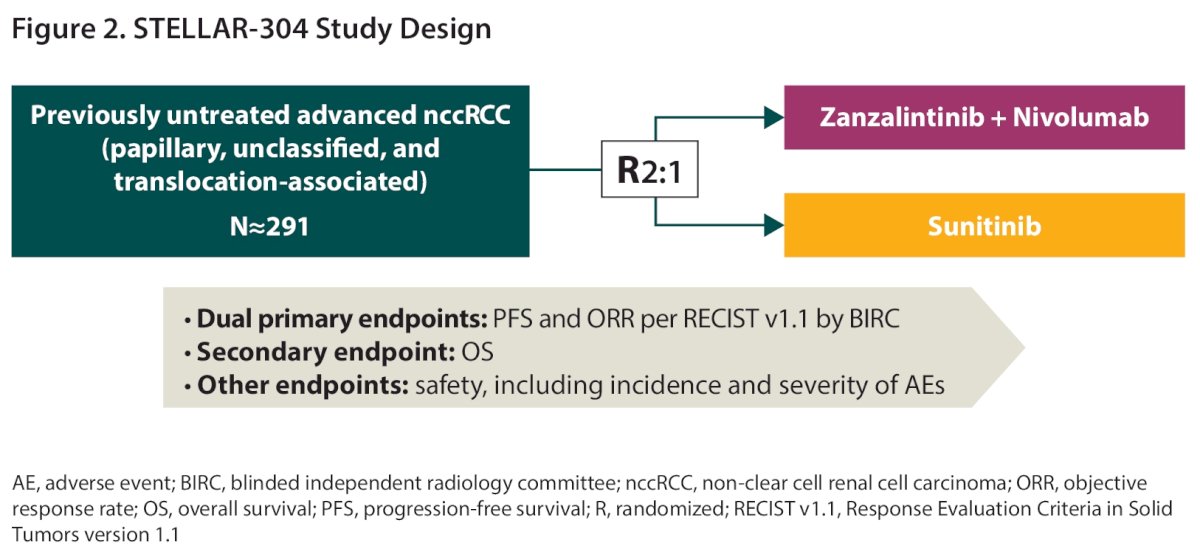
The dual co-primary endpoints are progression-free survival and objective response rate. Secondary endpoints include:
- Overall survival
- Safety
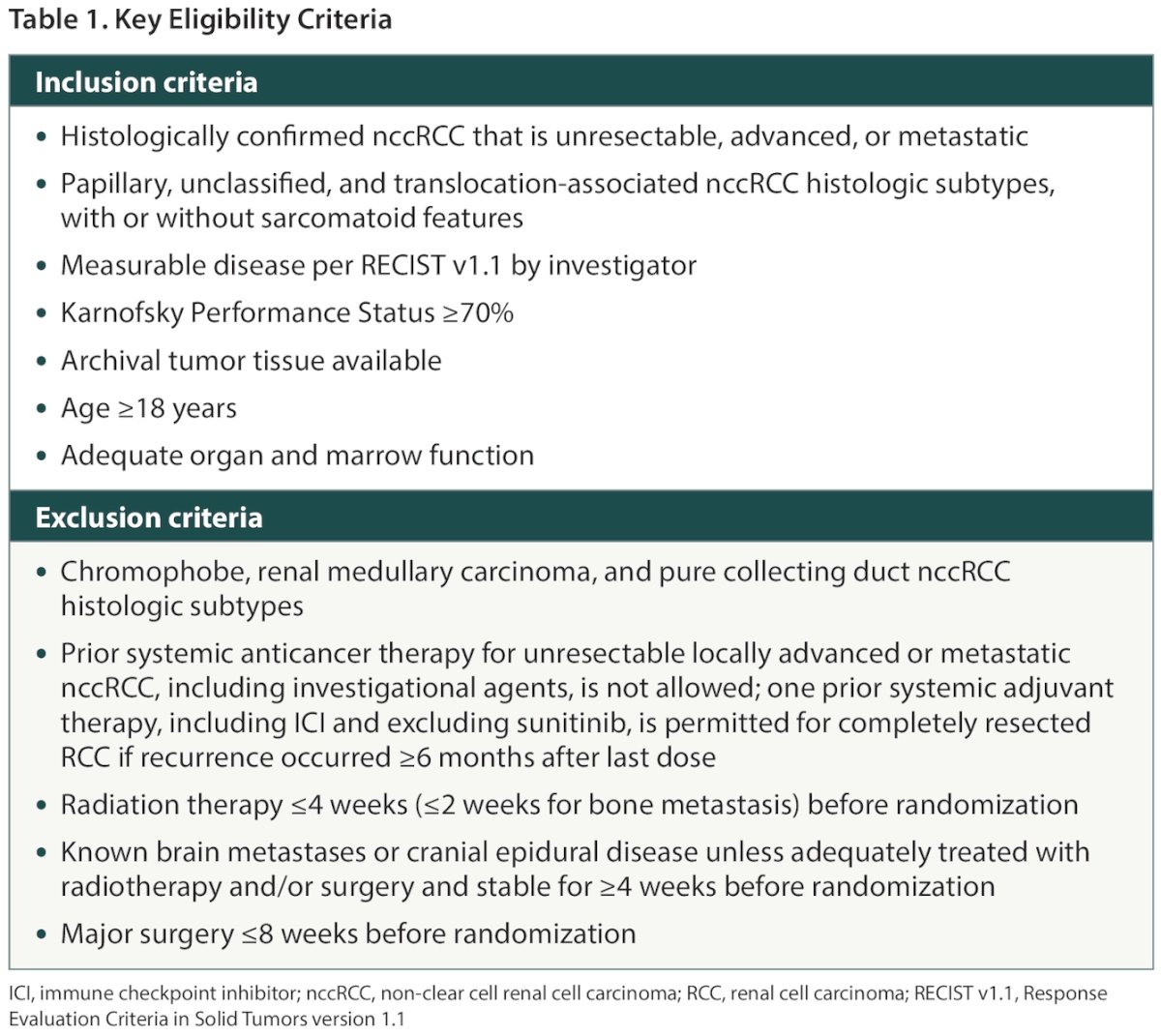
Patients with papillary, unclassified, or translocation-associated histologic subtypes, with or without sarcomatoid features, are eligible for enrollment (Table 1). Patients with chromophobe, renal medullary carcinoma, or pure collecting duct histologic subtypes are excluded.
The study is currently enrolling patients across sites in Europe, South America, North America, and the Asia-Pacific region.
Presented by: Sumanta K. Pal, MD, FASCO, Professor, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References:
- Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17(3): 378-88.
- Tannir NM, Jonasch E, Albiges L, et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol. 2016;69(5): 866-74.
- Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. 2018;6(1):9.
- Lee CH, Voss MH, Carlo MI, et al. Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. J Clin Oncol. 2022;40(21): 2333p41.
- Sharma M, et al. Ann Oncol. 2022;33(suppl_7):Abstract 481P.


