(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Oral Abstract Session: Genitourinary Cancer-Kidney and Bladder. Dr. Charlene Mantia presented a comprehensive analysis of survival states over 4 years in CheckMate 9ER comparing first-line nivolumab plus cabozantinib versus sunitinib in advanced renal cell carcinoma (aRCC).
Checkmate 9ER was a phase 3, randomized, open-label trial, we randomly assigned adults with previously untreated clear-cell, advanced renal-cell carcinoma to receive either nivolumab (240 mg every 2 weeks) plus cabozantinib (40 mg once daily) or sunitinib (50 mg once daily for 4 weeks of each 6-week cycle). This study met its primary endpoint of progression-free survival and showed that the combination of Nivolumab+ Cabozantinib doubled the median progression-free survival 16.6 months (95% CI 12.5 to 24.9) compared to sunitinib alone 8.3 months (95% CI, 7.0 to 9.7). (1) In this trial, treatment continued until disease progression, toxicity, or withdrawal of consent. Nivolumab treatment was halted at 24 months if patients were still on treatment.
Immunotherapy (IO) regimens have been associated with prolonged disease control even after treatment discontinuation, without the need for further anticancer therapy. In several trials evaluating IO combined with Tyrosine Kinase Inhibitors (TKIs), the IO component has been halted after 24 months from treatment initiation as per protocol. A prior analysis of first-line nivolumab + ipilimumab for advanced renal cell carcinoma (aRCC) in the CheckMate 214 study showed that at 42 months since randomization, 52% of patients receiving nivolumab plus ipilimumab and 39% of those receiving sunitinib in the intermediate/poor-risk category were still alive, with 18% and 5% surviving treatment-free, respectively. Notably, treatment-free survival (TFS) was twice as long compared to sunitinib in intermediate/poor-risk patients (6.9 vs. 3.1 months) and three times as long for favorable-risk patients (11.0 vs. 3.7 months) (2, 3). However, a pooled analysis of three trials of IO+TKI combination therapy compared to sunitinib showed a similar 30-month mean TFS (2.7 vs. 2.9 months), respectively. (4)
TFS and survival states for immune checkpoint inhibitor plus tyrosine kinase inhibitor (TKI) combination are and area of increasing interest. The researchers conducted an integrated, comprehensive partitioned survival analysis describing how patients spend their overall survival (OS) time: on/off treatment and with/without toxicity.
The graphic below illustrates the various time-to-event endpoints and their definitions used in partitioned survival analysis. Of note, the two areas highlighted in blue (TFS with and without toxicity) would be of particular interest for this analysis.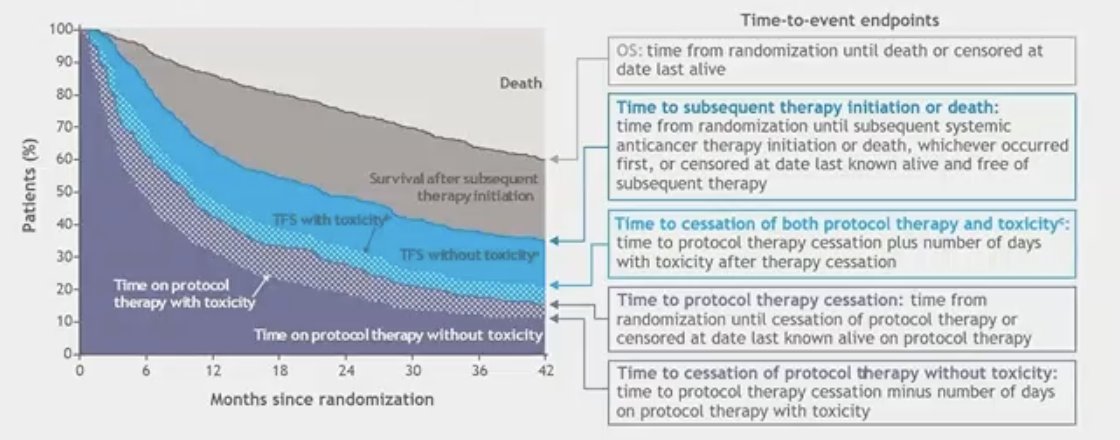
For this partitioned survival analysis, they included 651 patients with aRCC that were randomized in the CheckMate 9ER trial. The minimum follow-up was 48 months. For this analysis the investigators partitioned the area under the Kaplan–Meier (KM) OS curve into 3 survival states, that have been defined from randomization:
- Time on first-line protocol therapy
- Meantime with/without reported grade 2+ treatment-related adverse events (TRAEs)
- TFS: Time between firs-line protocol therapy cessation and second-line therapy initiation or death (difference between the two endpoints)
- Meantime with/without reported grade 2+ TRAEs
- Survival after subsequent systemic therapy (second line) initiation.
At four years post-randomization Nivolumab+Cabozantinib was associated with improved OS vs. sunitinib (49.2% vs 40.2%). Of the patients who were alive at four years 17.6% vs 4.7% were surviving treatment-free and 15.8% vs 8.2% were continuing first-line protocol therapy in the Nivolumab+Cabozantinib vs Sunitinib groups, respectively. Over the 48 months since randomization, the mean TFS (95% CI) was 2.4 (0.8–3.9) months longer after treatment with Nivolumab+Cabozantinib versus Sunitinib. At least half of TFS time was spent with toxicity in both treatment groups, resulting in difference in mean TFS without toxicity of 0.7 (95% CI −0.4 to 1.8) months.
Furthermore, the Nivolumab+Cabozantinib group spent a mean of 8.5 (95% CI 6.2–10.8) months more survival time on first-line protocol therapy, whereas the control group spent a mean of 6.5 (95% CI 4.4–8.6) months more survival time after second-line therapy initiation. 
In patients with IMDC intermediate/poor risk, Nivolumab + Cabozantinib was associated with improved OS compared to sunitinib (46.8% vs. 35.4%). At 48 months, 15.7% of patients in the Nivolumab + Cabozantinib group were continuing first-line protocol therapy, compared to 5.9% in the Sunitinib group. The mean TFS (95% CI) was 2.5 (0.8–4.3) months longer after treatment with Nivolumab + Cabozantinib, and the difference in mean TFS without toxicity was 0.6 (95% CI −0.4 to 1.8) months.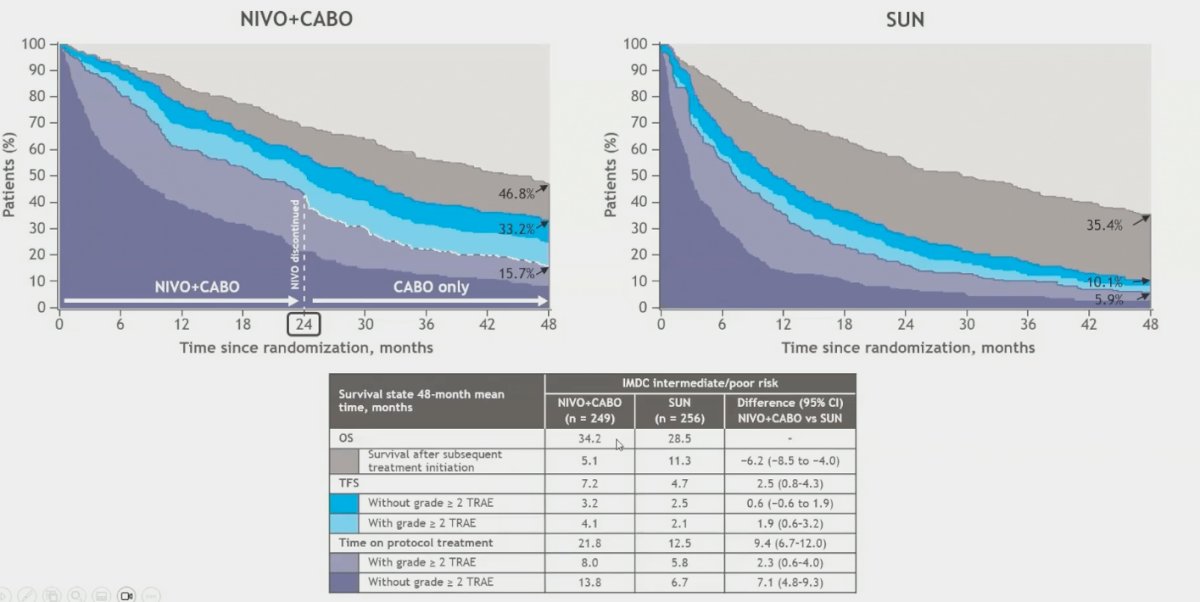
In subgroup analysis, Nivolumab + Cabozantinib was associated with a significant improvement in TFS across the majority of subgroups, including patients with aggressive disease (liver and bone metastasis), and irrespective of region (USA/Canada and Europe).
Dr. Mantia shared the Swimmers plot, illustrating the reasons for stopping protocol therapy and entering the TFS period. As depicted in the figure below, patients in yellow remain on therapy, those in green stop due to TRAEs, those in red stop for other reasons (consent withdrawal, treatment completion per protocol), and those in orange stop because of progressive disease (PD). Interestingly, the patients who discontinued treatment due to adverse events, toxicity, or other reasons had a longer TFS period.
In her closing remarks, Dr. Mantia underscored the following key messages:
- At four years post-randomization in the CheckMate 9ER study, there was a longer OS in patients assigned to Nivolumab + Cabozantinib compared to Sunitinib (49.2% vs. 40.2%).
- The longer OS achieved with Nivolumab + Cabozantinib was accompanied by 1.5 times longer mean time surviving treatment-free before second-line therapy compared to Sunitinib (difference of 2.4 months).
- The mean TFS difference was similarly longer for Nivolumab + Cabozantinib versus Sunitinib across most subgroups analyzed.
- TFS was more frequently observed after patients discontinued therapy for TRAEs, toxicity, or other reasons such as completion of therapy per protocol.
- This research highlights the importance of monitoring patient status after treatment discontinuation to understand the patient survival experience.
Presented by: Charlene Mantia, MD, Medical Oncologist, Dana-Farber Cancer Institute, Boston, Massachusetts.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References:
- Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, Suárez C, Hamzaj A, Goh JC, Barrios C, Richardet M, Porta C, Kowalyszyn R, Feregrino JP, Żołnierek J, Pook D, Kessler ER, Tomita Y, Mizuno R, Bedke J, Zhang J, Maurer MA, Simsek B, Ejzykowicz F, Schwab GM, Apolo AB, Motzer RJ; CheckMate 9ER Investigators. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021 Mar 4;384(9):829-841. doi: 10.1056/NEJMoa2026982. PMID: 33657295; PMCID: PMC8436591.
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, Porta C, Powles T, Donskov F, George S, Kollmannsberger CK, Gurney H, Grimm MO, Tomita Y, Castellano D, Rini BI, Choueiri TK, Leung D, Saggi SS, Lee CW, McHenry MB, Motzer RJ. First-line Nivolumab plus Ipilimumab Versus Sunitinib in Patients Without Nephrectomy and With an Evaluable Primary Renal Tumor in the CheckMate 214 Trial. Eur Urol. 2022 Mar;81(3):266-271. doi: 10.1016/j.eururo.2021.10.001. Epub 2021 Nov 5. PMID: 34750035; PMCID: PMC10202028.
- Regan MM, Jegede OA, Mantia CM, Powles T, Werner L, Motzer RJ, Tannir NM, Lee CH, Tomita Y, Voss MH, Plimack ER, Choueiri TK, Rini BI, Hammers HJ, Escudier B, Albiges L, Huo S, Del Tejo V, Stwalley B, Atkins MB, McDermott DF. Treatment-free Survival after Immune Checkpoint Inhibitor Therapy versus Targeted Therapy for Advanced Renal Cell Carcinoma: 42-Month Results of the CheckMate 214 Trial. Clin Cancer Res. 2021 Dec 15;27(24):6687-6695. doi: 10.1158/1078-0432.CCR-21-2283. Epub 2021 Nov 10. PMID: 34759043; PMCID: PMC9357269.
- Chang E, Zhou J, Song C, Gittleman H, Fernandes L, Weinstock C, Atkins MB, Agrawal S, Sridhara R, Gormley N, Tang S, Suzman DL, Amiri-Kordestani L, Kluetz PG, Pazdur R, Rini BI, McDermott DF, Regan MM. A Pooled Analysis of Treatment-Free Survival in Advanced Renal Cell Carcinoma. Clin Cancer Res. 2024 Feb 28. doi: 10.1158/1078-0432.CCR-23-3719. Epub ahead of print. PMID: 38416426.


