The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured an oral abstract session on kidney cancer, and a presentation by Dr. Laurence Albiges discussing a circulating kidney injury molecule-1 biomarker analysis in IMmotion010. In IMmotion010, adjuvant atezolizumab did not prolong investigator-assessed disease-free survival (primary endpoint) versus placebo after resection in patients with RCC (HR 0.93, 95% CI 0.75, 1.15; p = 0.50 [1]):

Heterogeneity in outcomes across clinical trials evaluating checkpoint inhibitors as adjuvant therapy in renal cell carcinoma suggests that there may be patient subpopulations that derive differential benefit from these agents. Additionally, biomarkers are needed to identify patients with minimal residual disease after resection who may have increased risk of recurrence. This exploratory analysis of circulating protein biomarkers was performed to identify high-risk patients with minimal residual disease who may show differential benefit from treatment with atezolizumab.
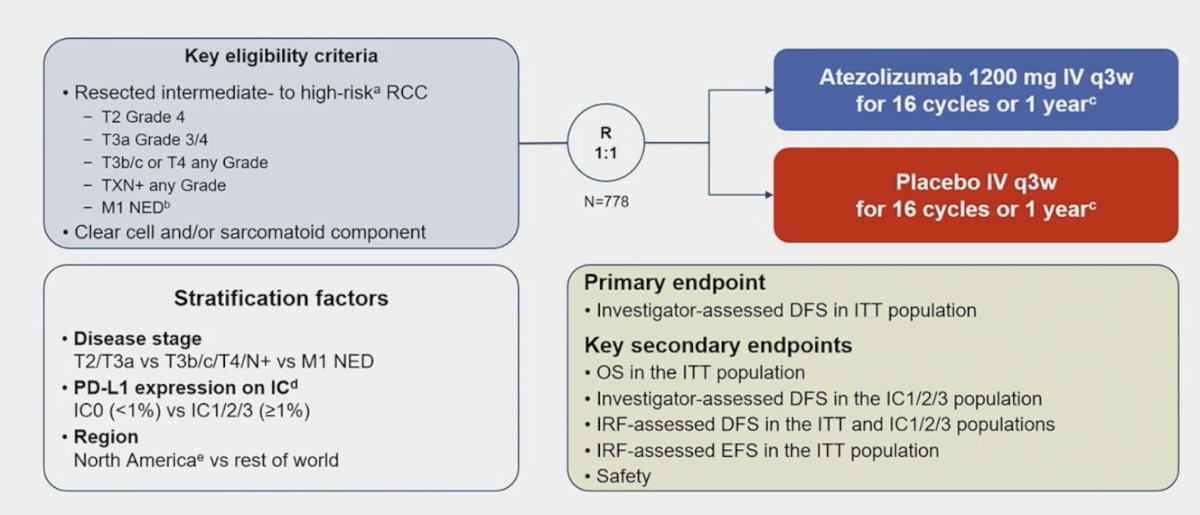
Patients with RCC with clear-cell or sarcomatoid component and increased recurrence risk post nephrectomy were randomized 1:1 to atezolizumab 1,200 mg or placebo IV q3w for 16 cycles or one year:
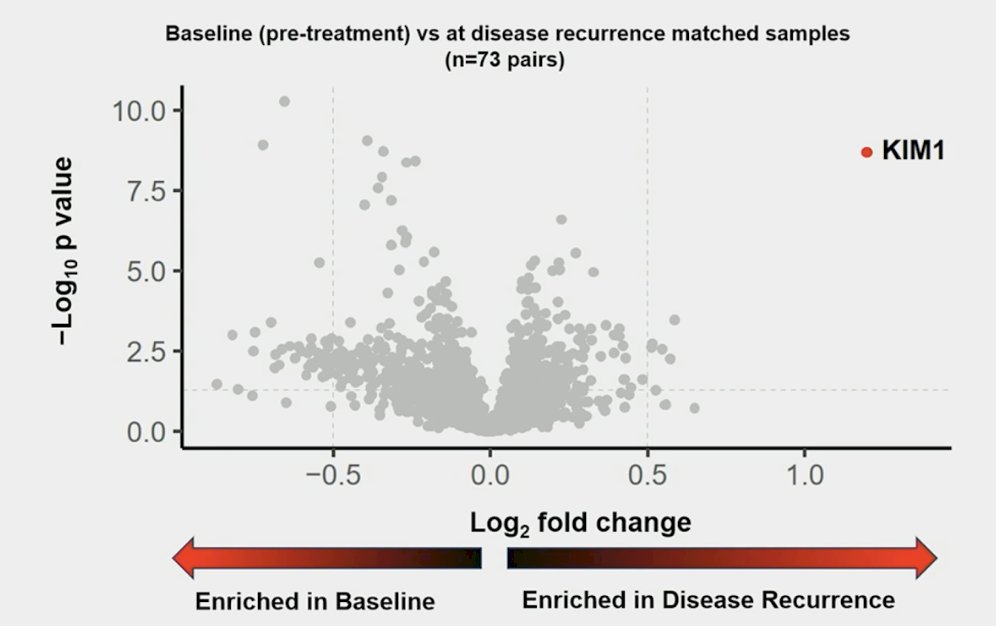
Part one of the circulating biomarker analysis scheme included a retrospective proteomics analysis was done with an affinity-based proximity extension assay panel of approximately 3,000 analytes to identify circulating proteins with differential abundance patterns in matched serum samples (baseline versus at recurrence). Circulating proteins with differential abundance patterns in matched baseline versus disease recurrence samples (n=73) were identified. Among these 73 patients with matched proximity extension assay, circulating kidney injury molecule-1 was identified as the most significantly enriched protein at recurrence versus baseline:
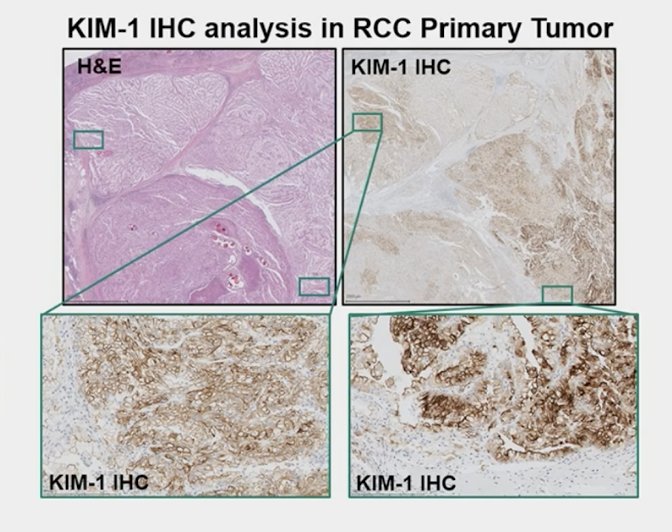
Kidney-injury molecule-1, a type 1 membrane glycoprotein, has been identified as a marker of unresected clear cell RCC and as a marker for early detection of RCC. In the ASSURE trial of adjuvant sunitinib, sorafenib, or placebo, higher levels of kidney injury molecule-1 in post-nephrectomy, pre-treatment plasma samples were associated with worse disease free and overall survival [2]. Kidney injury molecule-1 can be measured in plasma or serum and is stable under different storage conditions, suggesting suitability to serve as a peripheral blood circulating marker:
In Part 2 assessing the association of circulating kidney injury molecule-1 with disease free survival outcomes, a high sensitivity electrochemiluminescence assay was then used to evaluate levels of kidney injury molecule-1, in all available baseline and post-treatment serum samples. Outcomes in patients with kidney injury molecule-1 high (≥86 pg/mL) versus low status at baseline were analyzed:
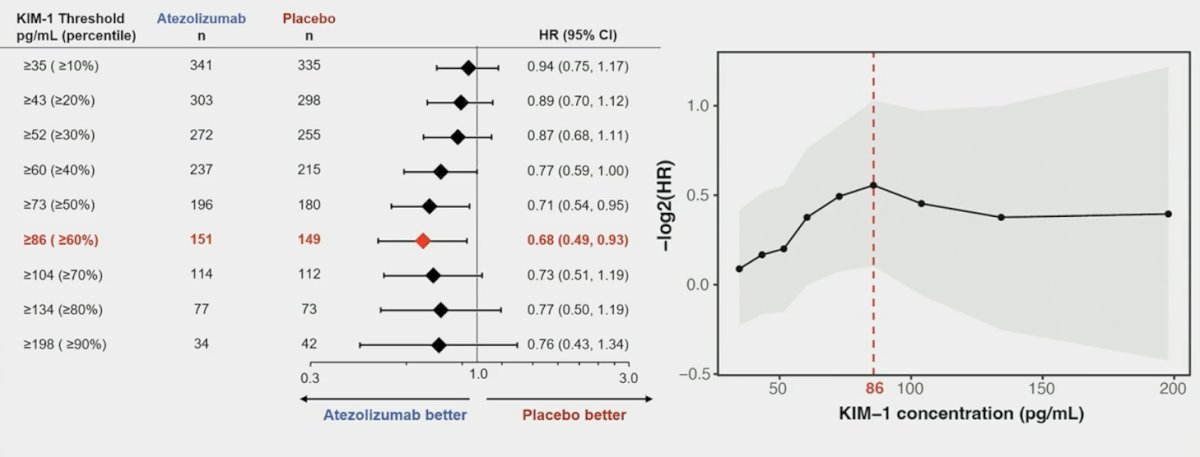
Among 778 patients enrolled in IMmotion010, 752 (97%) had baseline kidney injury molecule-1 data (high: 300 [40%]; low: 452 [60%]):

Kidney injury molecule-1–high status was associated with reduced disease-free survival, and patients with kidney injury molecule-1 high had better disease-free survival with atezolizumab versus placebo (HR 1.75, 95% CI 1.40-2.17):
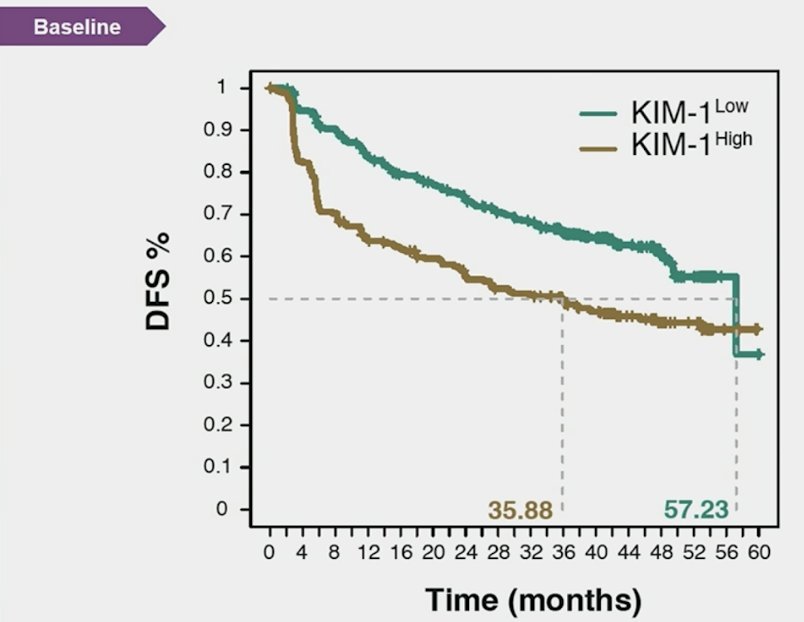
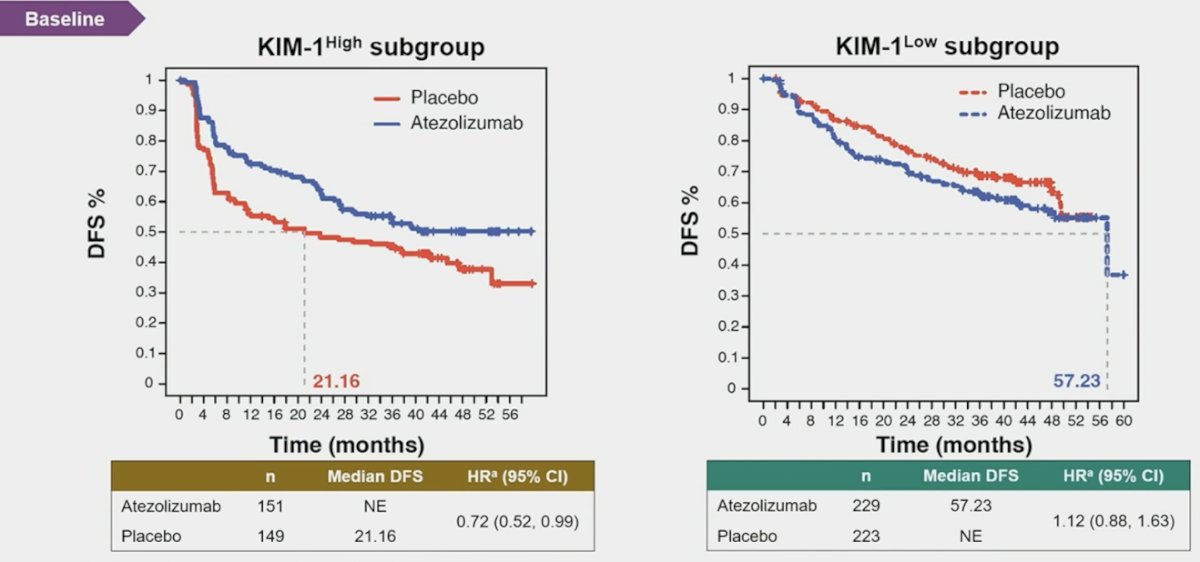
Moreover, atezolizumab improved disease free survival versus placebo in the baseline kidney injury molecule-1 high subgroup (HR 0.72, 95% CI 0.52-0.99), but not in the kidney injury molecule-1 low group:
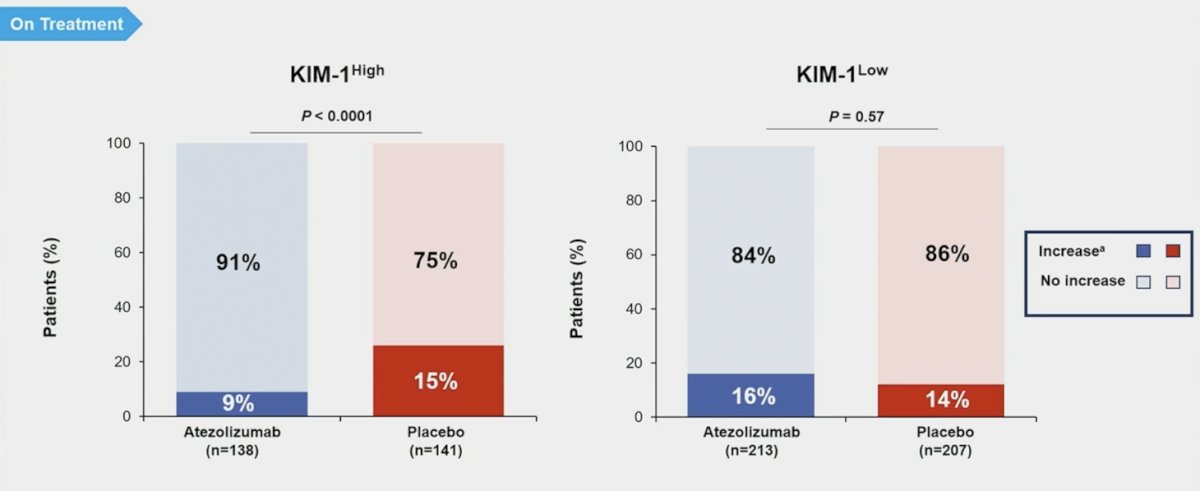
In the kidney injury molecule-1 high subgroup, patients were less likely to experience an on treatment increase in kidney injury molecule-1 levels with atezolizumab versus placebo treatment:
A ≥30% kidney injury molecule-1 increase was associated with worse disease-free survival in both kidney injury molecule-1–high (atezolizumab HR 1.68, 95% CI 0.77, 3.69; placebo HR 3.53, 95% CI 2.24, 5.58) and kidney injury molecule-1–low (atezolizumab HR 3.56, 95% CI 2.21, 5.75; placebo HR 3.22, 95% CI 1.81, 5.70) subgroups:

In patients with matched electrochemiluminescence samples (n = 103), median kidney injury molecule-1 levels were higher (p < 0.001) at recurrence (172 pg/mL) than at baseline (79 pg/mL):

Dr. Albiges concluded her presentation discussing a circulating kidney injury molecule-1 biomarker analysis in IMmotion010 with the following take home messages:
- In IMmotion010, high baseline serum kidney injury molecule-1 levels were associated with poorer prognosis but improved clinical outcomes with atezolizumab versus placebo
- Increased post-treatment kidney injury molecule-1 was associated with worse disease-free survival
- Additional validation studies are warranted to confirm the utility of circulating serum kidney injury molecule-1 in adjuvant RCC as a non-invasive biomarker for:
- Identification of minimal residual disease
- Predicting outcomes to checkpoint inhibitor treatment
- Longitudinal monitoring for disease recurrence
References:
- Pal SK, Uzzo R, Karam JA, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomized, double-blind, phase 3 trial. Lancet. 2022 Oct 1;400(10358):1103-1116.
Presenter: Laurence Albiges, Gustave Roussy, Université Paris Saclay, Villejuif, France
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


