The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Oral Abstract Session: Genitourinary Cancer-Kidney and Bladder. Dr. Robert Motzer presented the final overall survival (OS) analysis from the JAVELIN Renal 101 phase 3 trial comparing Avelumab + Axitinib vs. Sunitinib in patients with advanced renal cell carcinoma (aRCC).
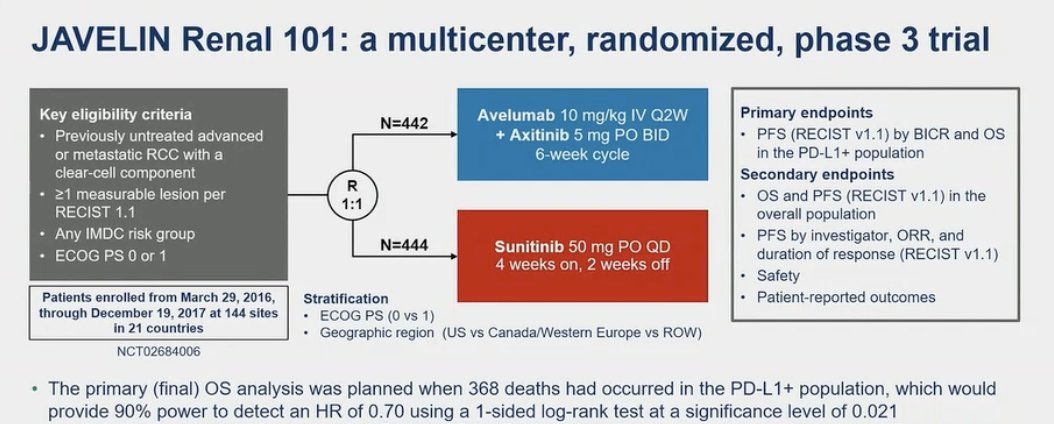
Avelumab + Axitinib was approved as first line treatment for patients with aRCC based on JAVELIN Renal 101 (NCT02684006). This was a phase 3 trial involving previously untreated patients with aRCC (any IMDC risk score). This trial compared avelumab plus axitinib with the standard-of-care at the time sunitinib. This trial randomly assigned patients in a 1:1 ratio to receive avelumab (10 mg per kilogram) intravenously every 2 weeks plus axitinib (5 mg) orally twice daily or sunitinib (50 mg) orally once daily for 4 weeks (6-week cycle). (1) The JAVELIN Renal 101 trial had two independent primary end points:
- Progression-free survival among patients with programmed death ligand 1 (PD-L1)–positive tumors.
- Overall survival in patients with (PD-L1)–positive tumors.
OS and PFS in the overall population were key secondary endpoints as well as overall response rate and safety. Of note, the primary final OS analysis was planned when 368 deaths had occurred in the PD-L1+ population, providing a power of 90% to detect a hazard ratio (HR) of 0.7. The trial design is outlined below:
JAVELIN Renal 101 previously met one of its primary endpoints by showing significantly longer progression-free survival (PFS) with avelumab + axitinib vs. sunitinib in patients with PD-L1+ tumors: median PFS 13.8 months vs. 7.2 months (HR 0.61; 95% CI 0.47 to 0.79; P<0.001), but also demonstrated longer median PFS in the overall population 13.8 months with avelumab plus axitinib, as compared with 8.4 months with sunitinib (HR 0.69; 95% CI, 0.56 to 0.84; P<0.001). However, OS data was immature at the time of previous analysis (at least 6 and 28 months of follow-up).
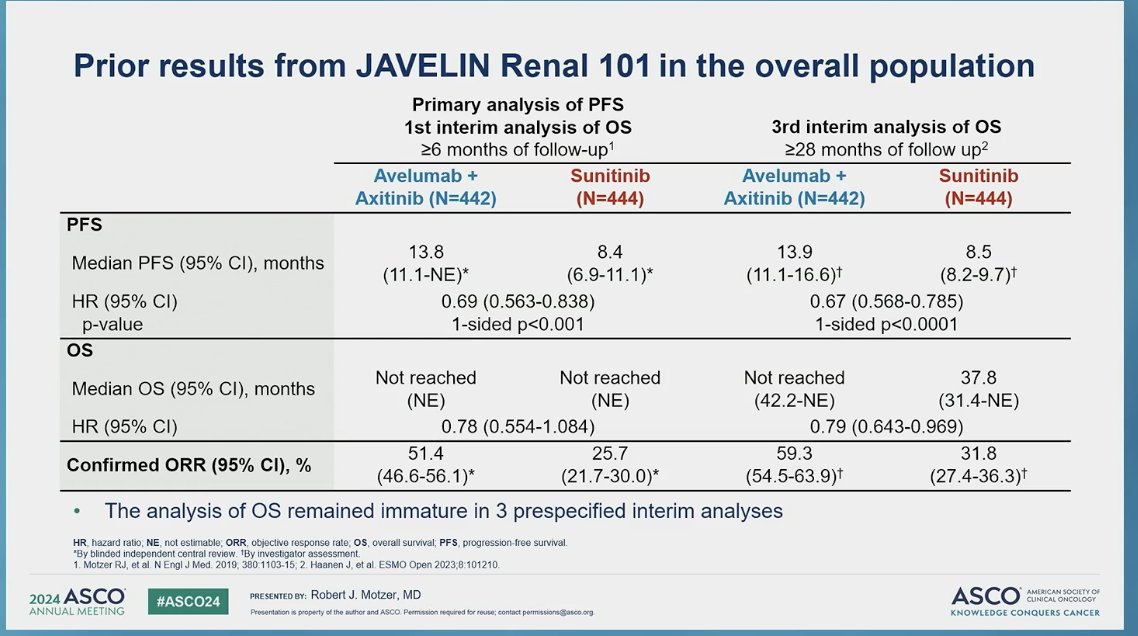
A total of 886 patients were randomized, 560 (63.2%) had PD-L1+ tumors. At data cutoff (August 31, 2023), the median follow-up in the avelumab + axitinib and sunitinib arms was 73.7 and 73.6 months, respectively. Importantly the follow-up was ≥68 months for all patients.
The median OS for the PD-L1+ population was 43.2 (95% CI 36.5-51.7) months for those treated with avelumab + axitinib compared to 36.2 (95% CI 29.8-44.2) months for Sunitinib. This was not significant (HR 0.86 95% CI 0.701-1.057, p=0.076). For the overall population the median OS was 44.8 (95% CI 39.7-51.1) months for those treated with avelumab + axitinib compared to 38.9 (95% CI 31.4-45.2) months for Sunitinib (HR 0.88 95% CI 0.749-1.039, p=0.067).

In the subgroup analysis for OS in the overall population, the outcome favored avelumab + axitinib in male and IMDC poor-risk subgroups only.
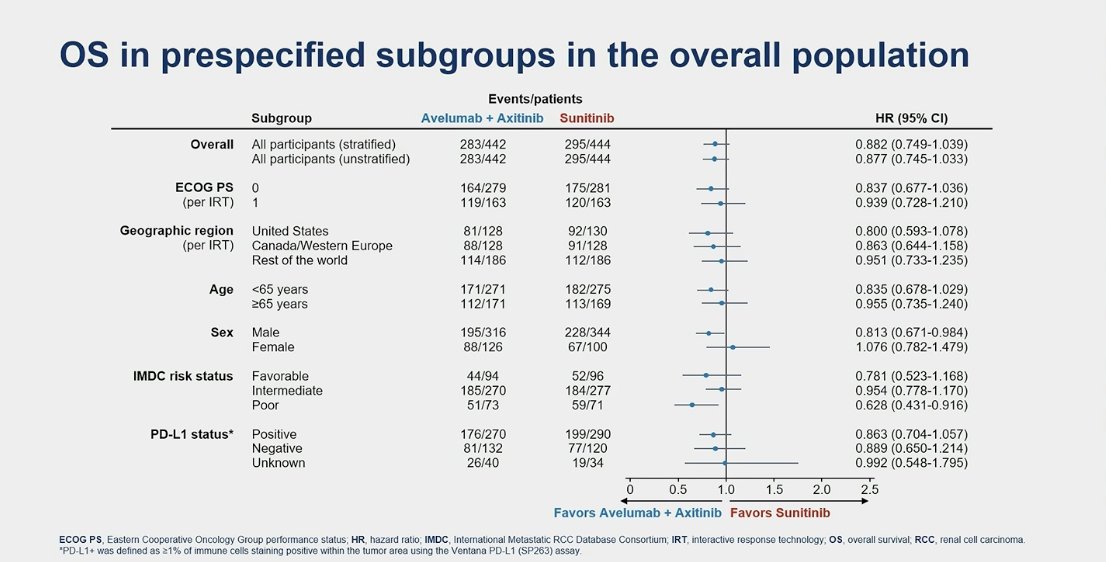
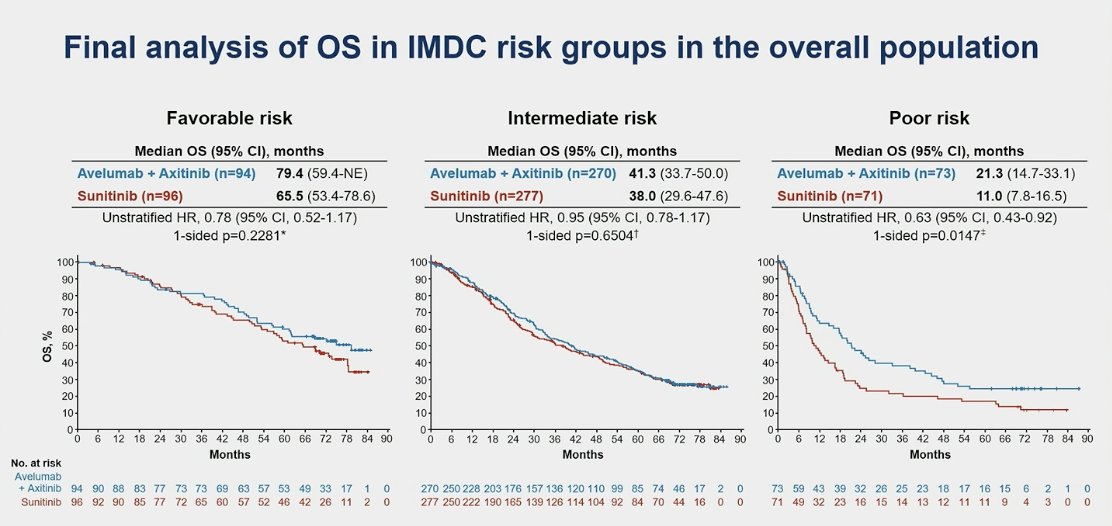
When stratifying by IMDC risk groups, OS was still not significantly better in the favorable or intermediate risk groups. However, in the poor risk group, the median OS was 21.3 months in the avelumab + axitinib group compared to 11.0 months for sunitinib (HR 0.63, 95% CI 0.43-0.92, p=0.015).
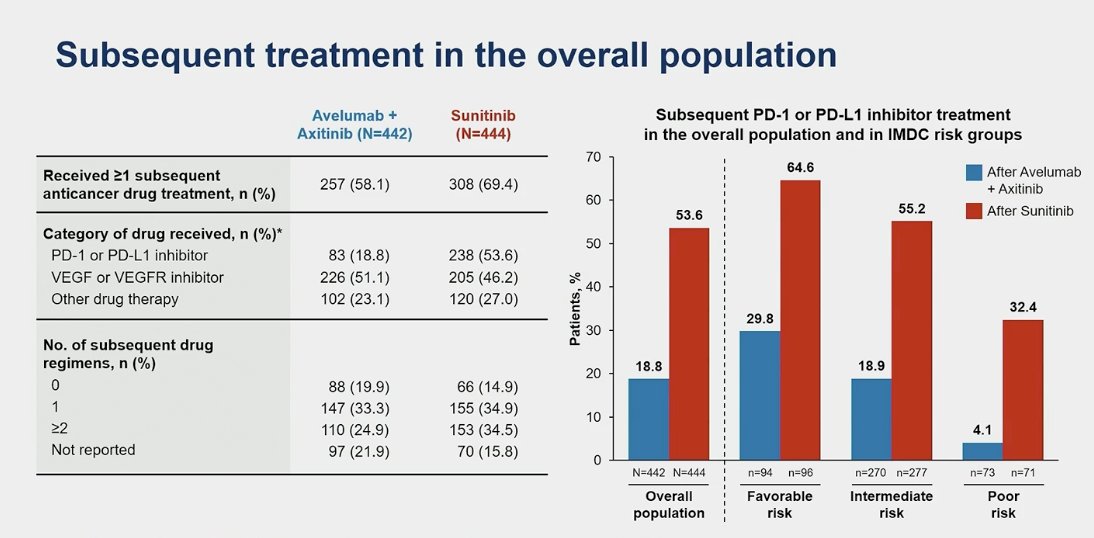
Second-line therapy was received by 58.1% of patients in the avelumab + axitinib arm and 69.4% in the sunitinib arm. This included a PD-L1 inhibitor in 18.8% of the avelumab + axitinib arm and 53.6% of the sunitinib arm. Additionally, more than double (65% vs. 30%) the number of patients in the favorable risk group and more than triple (55.2% vs. 19%) in the intermediate risk group received subsequent treatment in the sunitinib arm compared to the avelumab + axitinib arm.
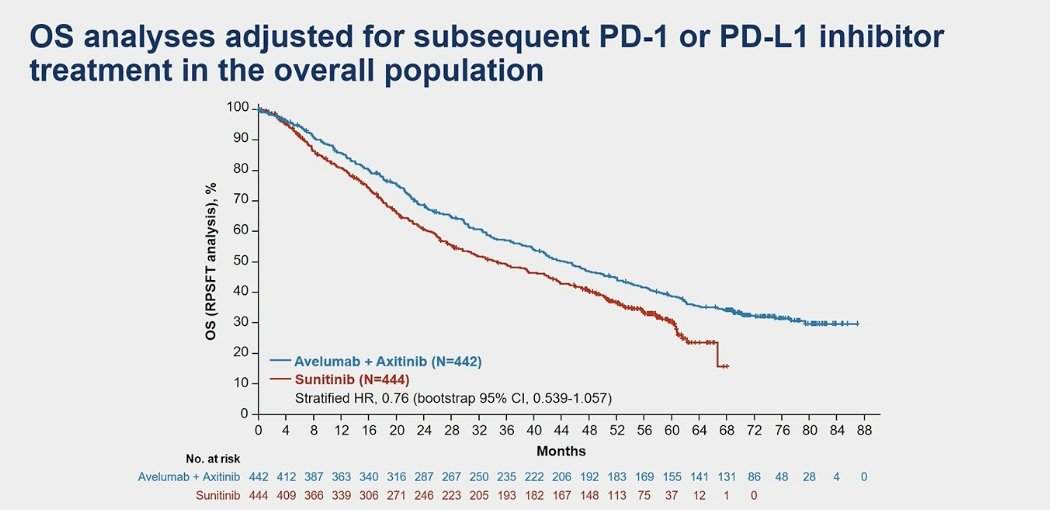
In an exploratory analysis, after adjusting for subsequent PD1 or PD-L1 treatment in the overall population, there was still no significant benefit associated with avelumab + axitinib for OS (sHR 0.76, 95% CI 0.539-1.057).
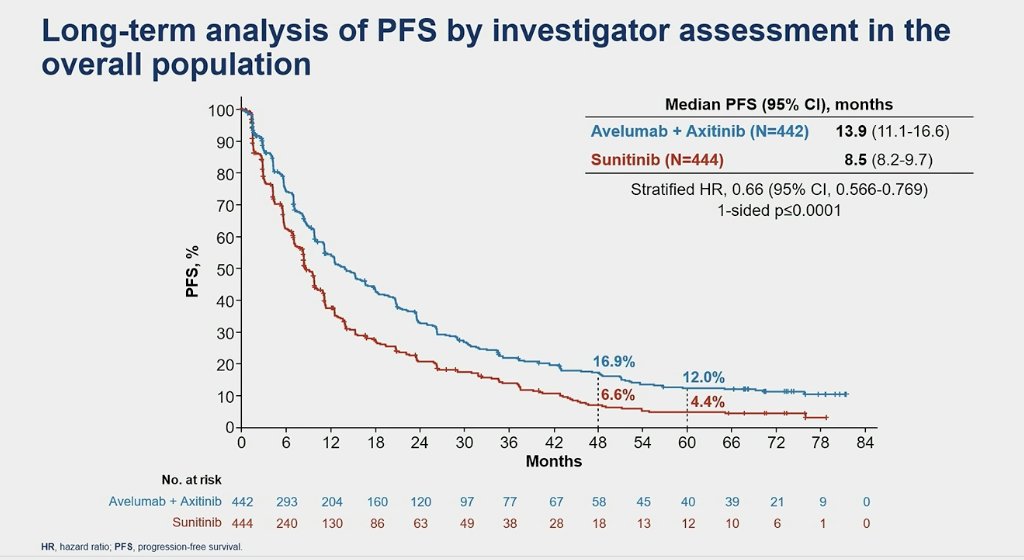
Furthermore, in this updated analysis of JAVELIN Renal 101, treatment with avelumab + axitinib was associated with a significantly longer PFS versus sunitinib, both in the PD-L1+ population (HR 0.57 95% CI 0.469-0.697, p<0.0001) and the overall population (HR 0.66 95% CI 0.566-0.769, p<0.0001).
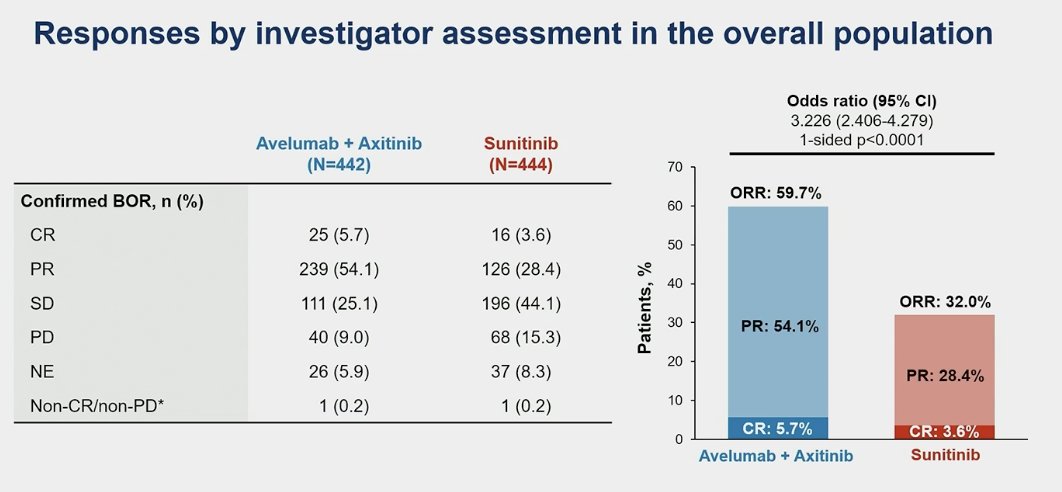
The overall response rate (ORR) of avelumab + axitinib was 64.8% (95%CI 58.8-70.5) in the PD-L1+ population compared to 31.4% (95% CI 26.1-37.1) in the sunitinib group. Similarly in the overall population the ORR for avelumab + axitinib was almost double 59.7 vs. 32% in the sunitinib arm.
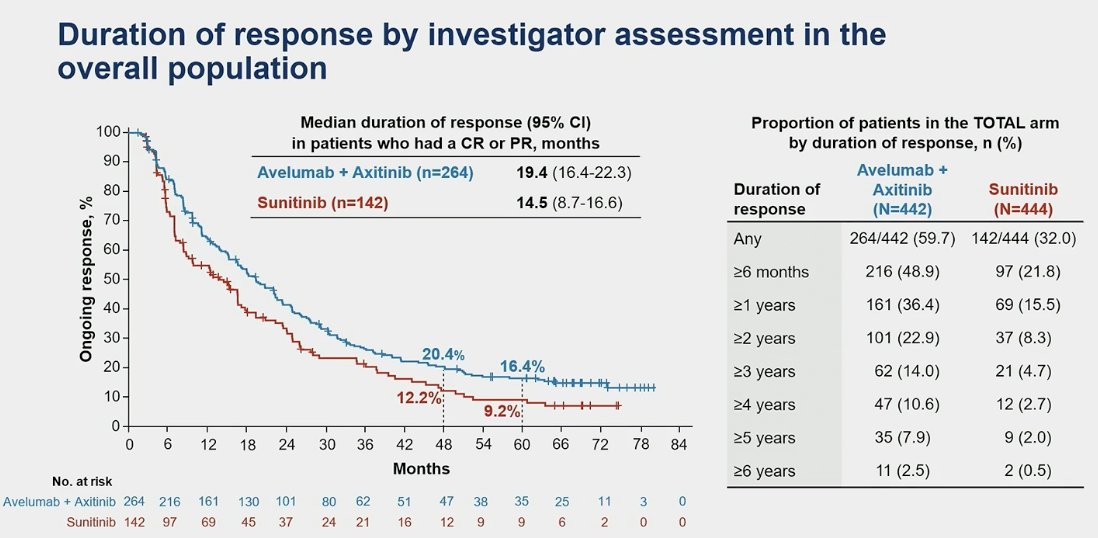
The median duration of response was 19.4 months for the IO+TKI combination vs. 14.5 months in the sunitinib arm.
Grade ≥3 treatment-related adverse events (TRAEs) occurred in 66.8% of patients in the avelumab + axitinib arm compared to 61.5% in the sunitinib arm. The most common TRAEs were diarrhea, fatigue, and hand-foot syndrome, most of which were Grade 1/2, except for hypertension (HTN).
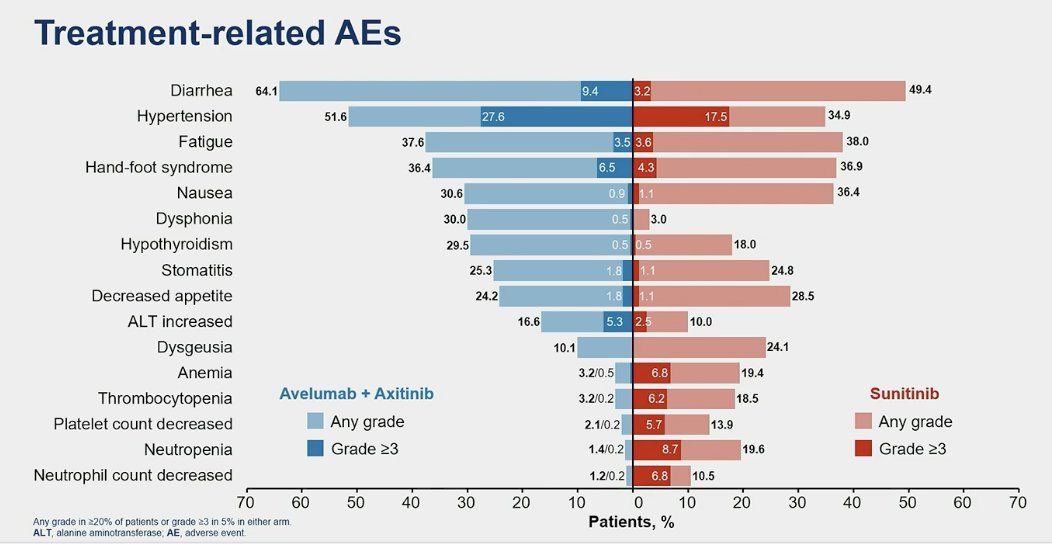
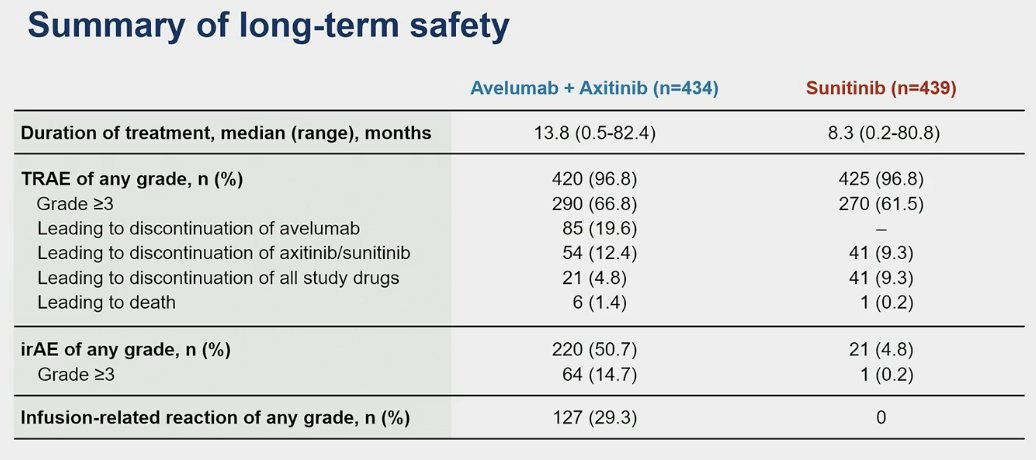
TRAEs leading to treatment discontinuation were observed in 19.6% for avelumab alone and 5% for both drugs (avelumab + axitinib). In the sunitinib arm, TRAEs leading to treatment discontinuation occurred in 9% of the patients.
Dr Motzer concluded his presentation with the following take home messages:
- JAVELIN Renal 101 trial provides the longest follow-up for any IO +TKI combination reported to date
- OS analyses favored avelumab + axitinib vs sunitinib but did not reach statistical significance.
- PFS was longer with avelumab + axitinib vs sunitinib
- ORR was almost double for avelumab + axitinib and responses were durable in a subset of patients
- Final analysis results confirm the long-term efficacy and manageable safety profile of avelumab + axitinib treatment in patients with aRCC
Presented By:
-Dr. Robert J. Motzer, MD, Genitourinary Medical Oncologist at Memorial Sloan Kettering Cancer Center
Written By: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References
- Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, Kollmannsberger C, Gravis-Mescam G, Uemura M, Lee JL, Grimm MO, Gurney H, Schmidinger M, Larkin J, Atkins MB, Pal SK, Wang J, Mariani M, Krishnaswami S, Cislo P, Chudnovsky A, Fowst C, Huang B, di Pietro A, Albiges L. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020 Aug;31(8):1030-1039. doi: 10.1016/j.annonc.2020.04.010. Epub 2020 Apr 25. PMID: 32339648; PMCID: PMC8436592.


