(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured an oral abstract session on kidney cancer, and a presentation by Dr. Toni Choueiri discussing biomarker analyses in patients with advanced renal cell carcinoma from the phase 3 CLEAR trial. In the primary analysis of CLEAR, lenvatinib + pembrolizumab significantly improved efficacy versus sunitinib in treatment-naïve patients with advanced renal cell carcinoma.1 These results were also confirmed at the final prespecified overall survival analysis.2 At the ASCO 2024 annual meeting, Dr. Choueiri and colleagues reported the biomarker analyses from CLEAR.
The trial design for CLEAR is highlighted as follows: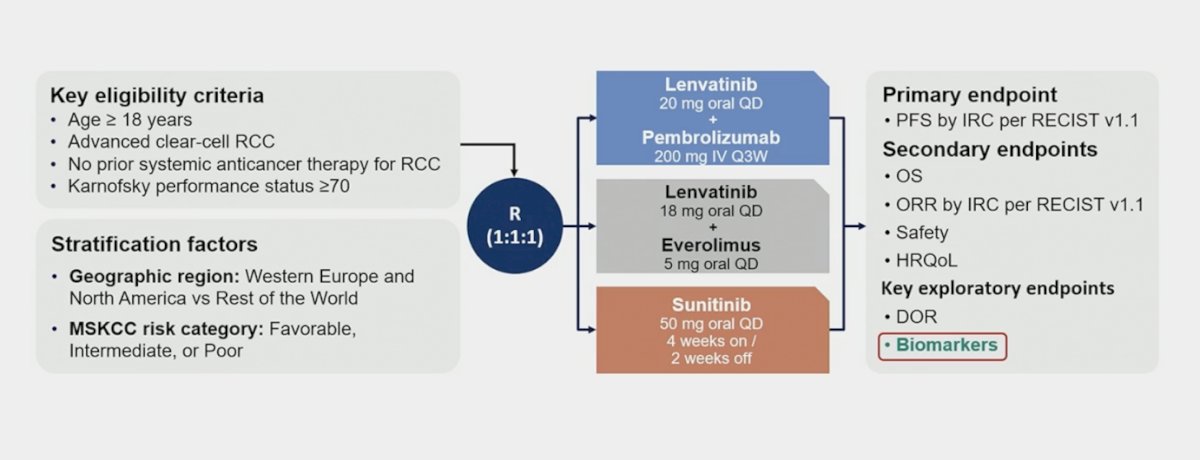
PD-L1 IHC 22C3 pharmDx and NGS assays (ImmunoID NeXT platform: WES and RNA-Seq) were performed on archival tumor specimens. To identify somatic alterations including mutations and copy-number variations, paired PBMC samples were used as reference: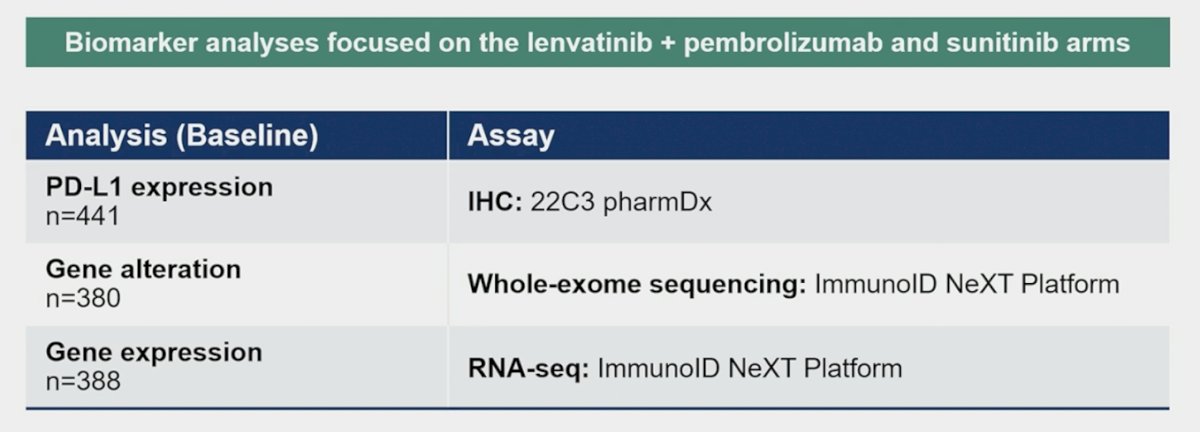
For RNA-Seq/IHC-derived analyses, a continuous value analysis was performed adjusting by KPS score for:
- Each gene-signature score (T-cell inflamed gene-expression profile, and non-gene-expression profile signatures including proliferation, angiogenesis, hypoxia, MYC, WNT, and other signatures [3]) versus best overall response;
- Non-gene-expression profile signatures versus best overall response adjusted by gene-expression profile
- PD-L1 CPS vs best overall response. Cutoff analyses were performed for biomarkers that showed significant association in the continuous value analysis.
Cutoff values (1st tertile of gene-expression profile, or median of non-gene-expression profile, signatures) were determined based on combined lenvatinib + pembrolizumab and sunitinib arms. WES analyses were descriptively summarized if TMB/INDEL burden and mutation status of key RCC driver genes were associated with best overall response.
There were no notable differences in baseline characteristics and tumor responses in biomarker analysis sets versus the intention to treat population: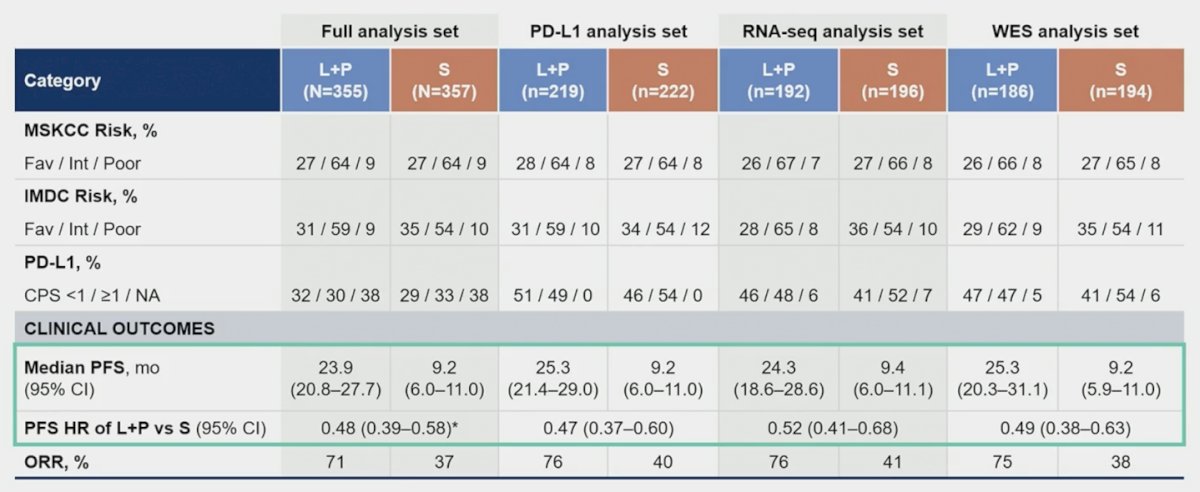
In the lenvatinib + pembrolizumab arm, the continuous gene-expression profile signature score was not associated with best overall response: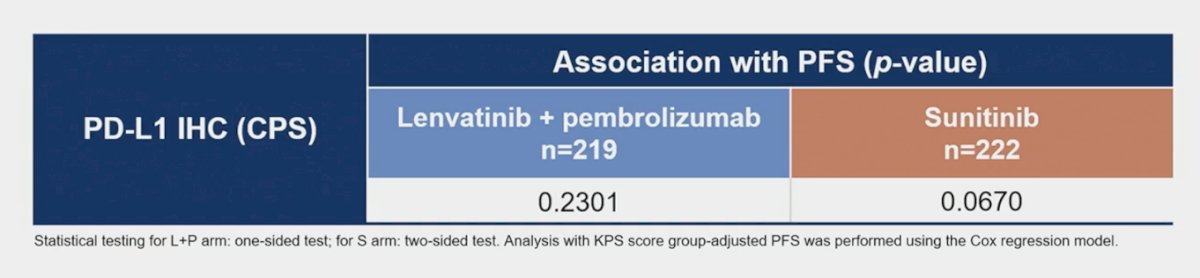
Progression-free survival was higher with lenvatinib + pembrolizumab vs sunitinib, regardless of the deleterious mutation status of BAP1, VHL, PBRM1, SETD2, and KDM5C, which are frequently mutated genes in RCC: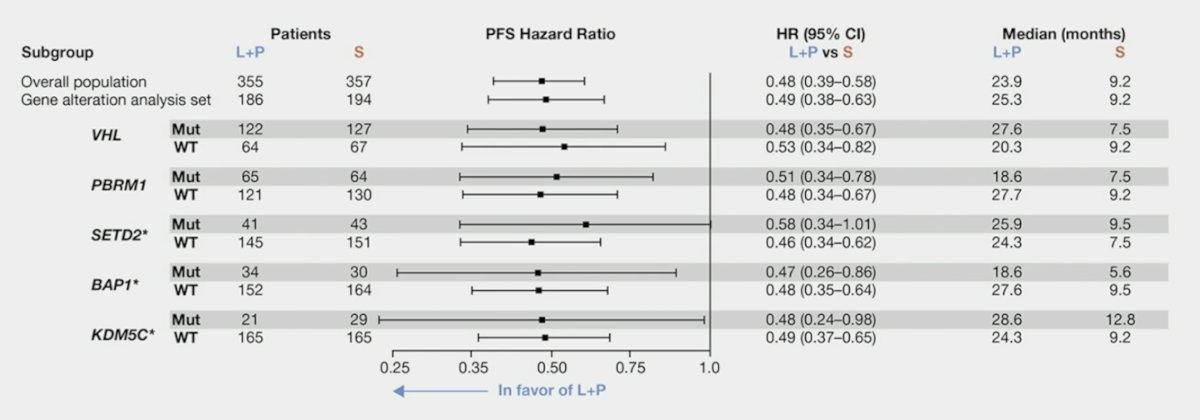
Gene signature scores were not associated with PFS outcomes for the lenvatinib + pembrolizumab arm:
Lenvatinib + pembrolizumab showed longer progression-free survival versus sunitinib regardless of signature-high and signature-low subgroups:
Objective response rate was also higher with lenvatinib + pembrolizumab vs sunitinib, regardless of the deleterious mutation status of BAP1, VHL, PBRM1, SETD2, and KDM5C: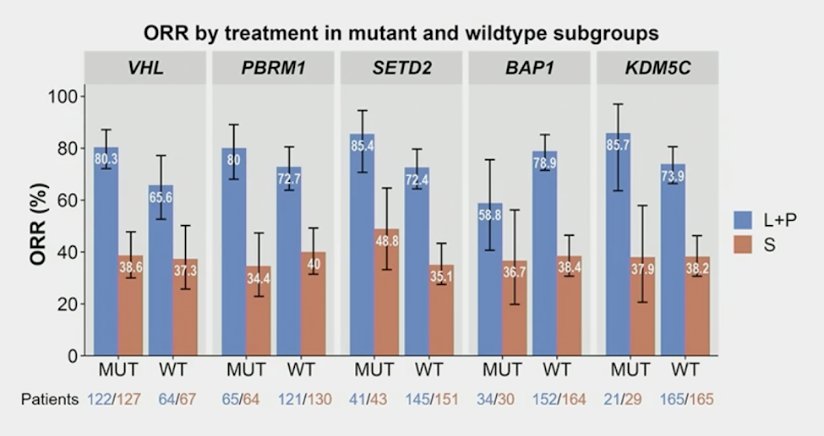
Tumors of patients with favorable and intermediate risk were enriched in the angiogenesis and angiogenesis/stromal clusters, and tumors of patients with poor risk were enriched in the proliferative cluster. Tumors of patients that were PD-L1+ were enriched in the immune/proliferative cluster: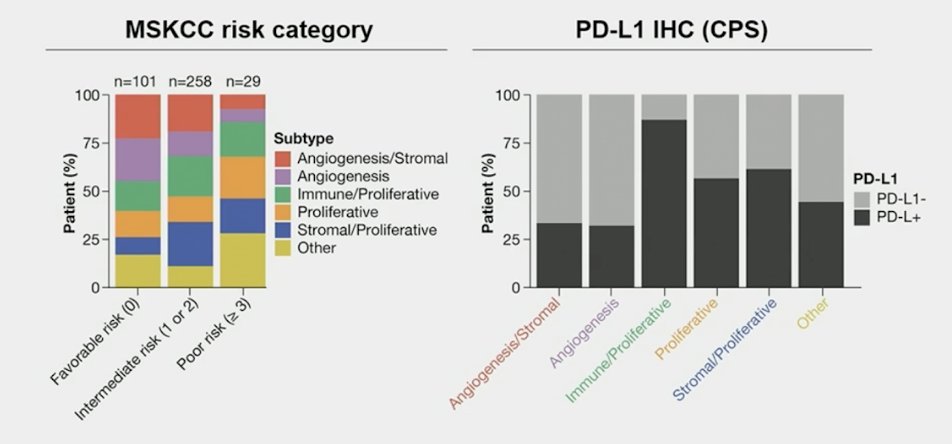
The objective response rates for the gene-expression profile-high and -low groups were 46.9% (95% CI 38.1-55.9) and 28.8% (95% CI 18.3-41.3), respectively. The angiogenesis signature was positively associated with best overall response (2-sided test, significance criteria 0.1; FDR-adjusted p = 0.046/0.088 with/without adjustment by gene-expression profile signature score, respectively). The objective response rates for the angiogenesis-high and -low groups were 52.1% (95% CI 41.6-62.5) and 30.4% (95% CI 21.7-40.3), respectively. Taken together, lenvatinib + pembrolizumab had numerically higher tumor response, as well as longer progression-free survival across all molecular subtypes: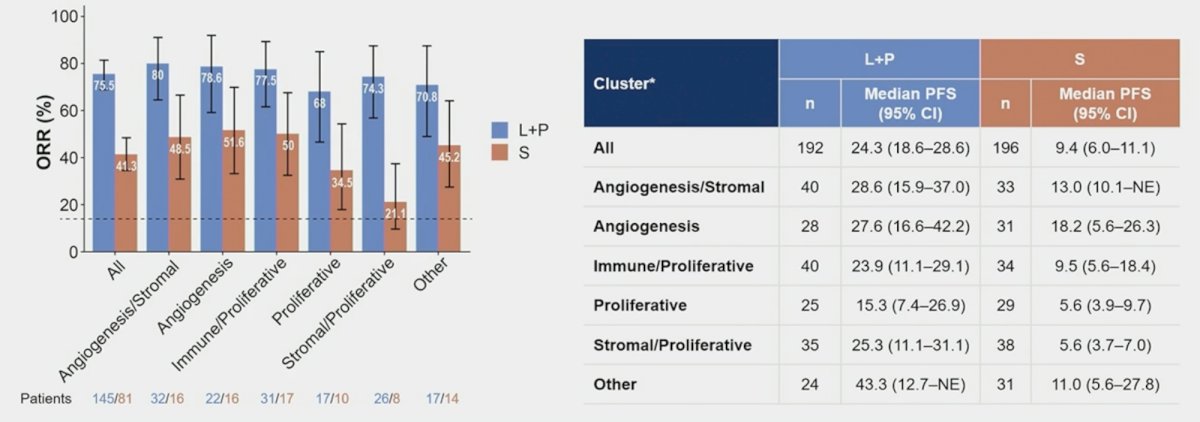
Dr. Choueiri concluded his presentation discussing biomarker analyses in patients with advanced renal cell carcinoma from the phase 3 CLEAR trial with the following take-home messages:
- Lenvatinib + pembrolizumab showed clinical benefit over sunitinib in patients with advanced renal cell carcinoma regardless of biomarker subtypes
- The progression-free survival analysis of lenvatinib + pembrolizumab confirms the high level efficacy of the combination irrespective of PD-L1 CPS
- Lenvatinib + pembrolizumab showed longer progression-free survival and improved objective response rate versus sunitinib regardless of RCC driver gene mutation status or gene signature
- Lenvatinib + pembrolizumab demonstrated numerically higher tumor response and longer progression-free survival than sunitinib across all molecular subtypes
Presenter: Toni K. Choueiri, MD, Jerome and Nancy Kohlberg Professor of Medicine, Harvard Medical School, Attending Physician, Solid Tumor Oncology, Director, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, Boston MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021 Apr 8;384(14):1289-1300.
- Motzer RJ, Porta C, Eto M, et al. Lenvatinib plus pembrolizumab versus sunitinib in first-line treatment of advanced renal cell carcinoma: Final prespecified overall survival analysis of CLEAR, a phase III study. J Clin Oncol. 2024 Apr 10;42(11):1222-1228.
- Cristescu R, Nebozhyn M, Zhang C, et al. Transcriptomic determinants of response to pembrolizumab monotherapy across solid tumor types. Clin Cancer Res. 2022 Apr 14;28(8):1680-1689.


