- Would I benefit from adjuvant therapy?
- What is my risk of recurrence?
In the metastatic setting, important questions that may be answered by biomarkers include:
- Do I need systemic therapy?
- What treatment should I get?
- Do I need combination therapy?
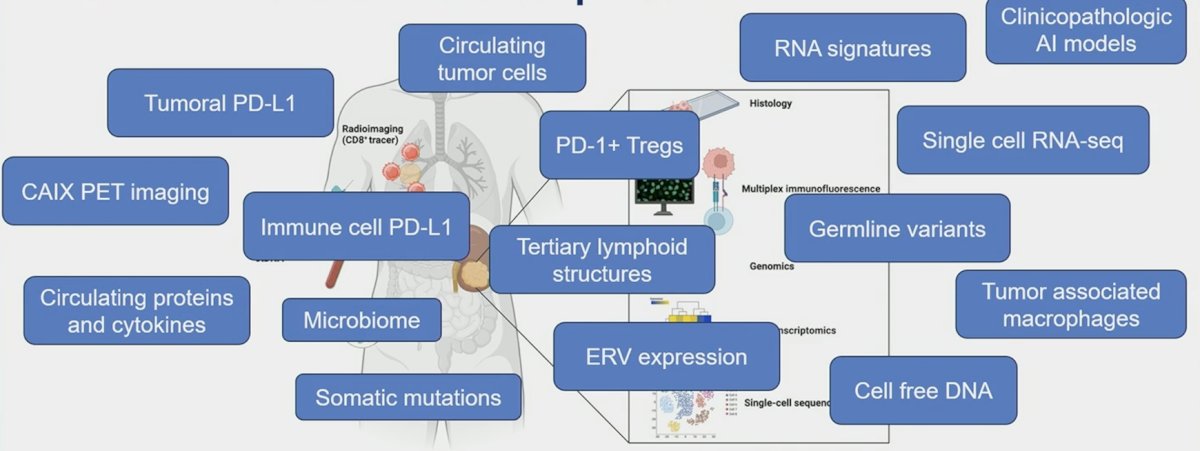
To date, many novel biomarkers have advanced our understanding of RCC biology, including histology, multiplex immunofluorescence, genomics, bulk transcriptomics, and single-cell sequencing:

However, individualized biomarker directed therapy remains elusive in clinical practice:
The two abstracts presented by Dr. Choueiri and Dr. Rini in the metastatic RCC setting aim to answer the question: Can we identify subgroups which benefit more from sunitinib versus IO + TKI combination therapy? To do this they assess PD-L1 expression, tumor mutations (WES), and gene expression changes (RNA-seq). For PD-L1 expression, does expression selectively identify patients who benefit from immunotherapy? PD-L1 has historically had limited usefulness for decision making in RCC, as highlighted by the following table:

However, updated data continues to show that immune checkpoint inhibitor doublets improve outcomes regardless of PD-L1 expression:

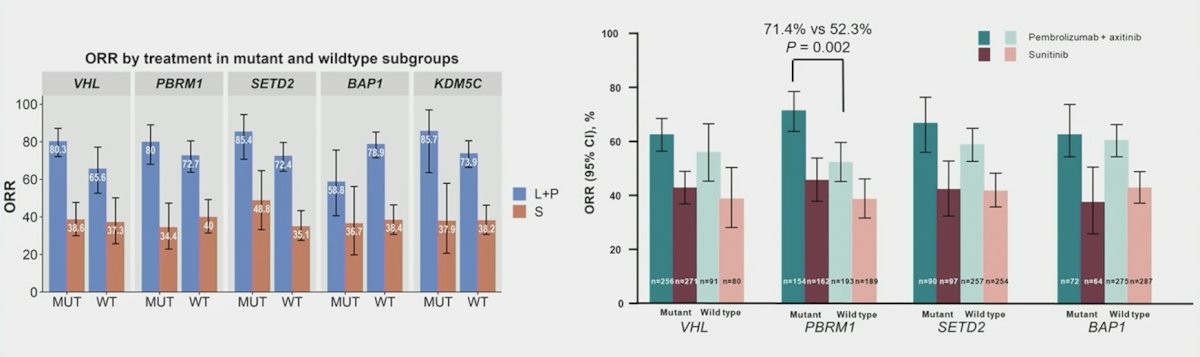
For tumor mutations, do specific tumor mutations predict benefit from sunitinib versus IO + TKI combination therapy? Historically, tumor mutations in RCC have included key clear cell RCC mutations that are co-localized on chromosome 3p. These include (i) VHL mutations, which are pathogenic in clear cell RCC and drive hypoxia response genes, (ii) PBRM1 mutations that affect chromatin remodeling and are associated with PD-1 response in some tumors, and (iii) SETD2 and BAP1, which control the histone code and DNA repair and are associated with prognosis in clear cell RCC. Dr. Xu notes that in both CLEAR and KEYNOTE-426, combination therapies improve outcomes across mutational status subgroups:
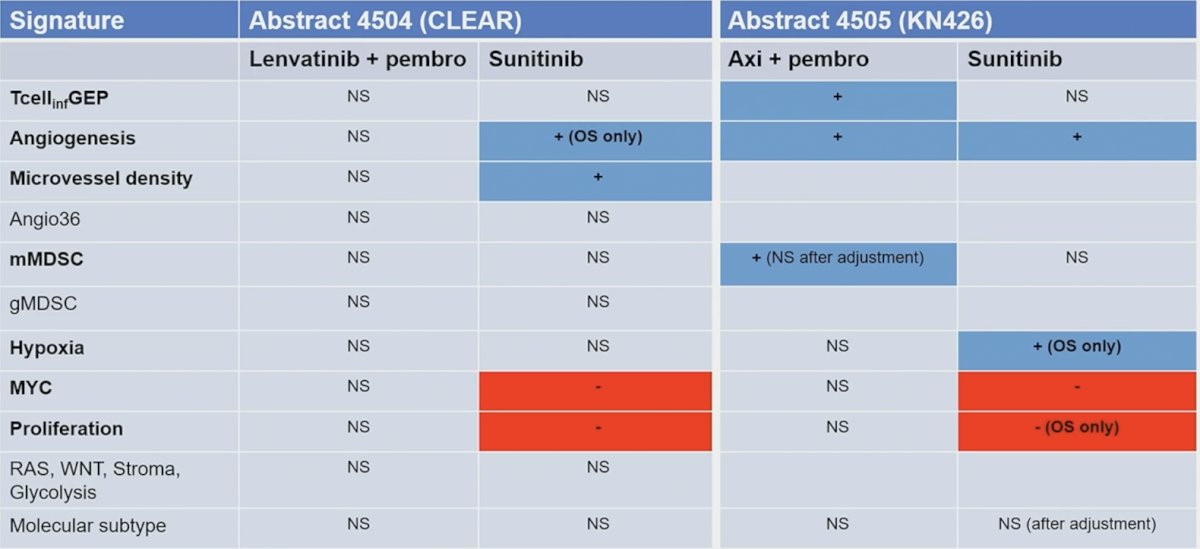
For gene expression changes, do transcriptomic signatures and molecular subgroups predict for benefit from sunitinib versus IO + TKI combination therapy? By way of background, transcriptomic signatures were derived from RCC trials (IMmotion150/151, JAVELIN Renal 101), but also there are tumor agnostic signatures, including T-cell inflamed gene expression profile values, angiogenesis, hypoxia, glycolysis, proliferation, MYC, RAS, gMDSC, mMDSC, and EMT. Importantly, Dr. Xu notes that signatures have overlapping biology, and some transcriptomic biomarkers are associated with differential progression free survival and overall survival:
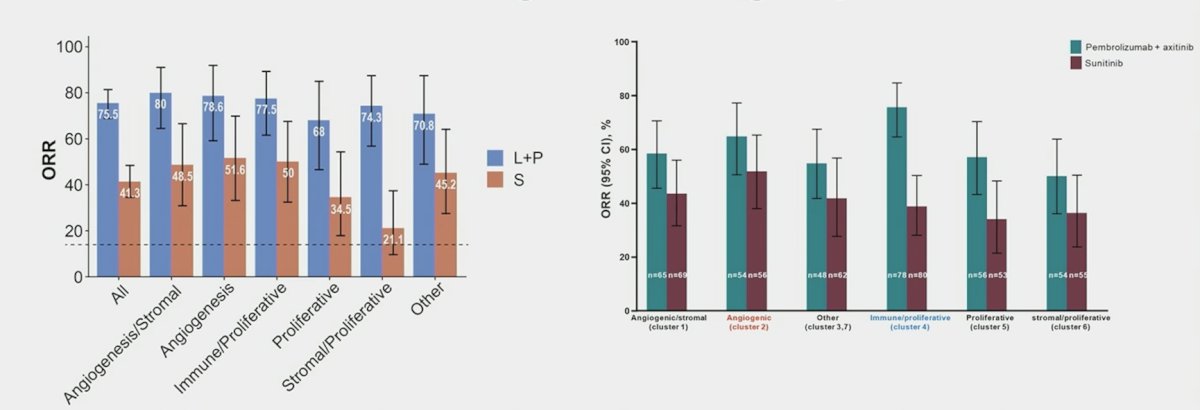
While some clusters benefit more, these data don’t justify denying Io + TKI combination to any subgroup in routine clinical practice:
Dr. Xu highlighted that there has been prospective evaluation of transcriptomic subtypes in RCC, namely in the BIONIKK study and the OPTIC RCC study. Dr. Xu then provided the following conclusions for biomarkers in CLEAR and KEYNOTE-426:
- Combination therapy (lenvatinib + pembrolizumab, axitinib + pembrolizumab) have known superiority to single agent sunitinib in the intention to treat population
- Despite these intriguing signals, combination therapy continues to perform better than single agent sunitinib across genetic and molecular subgroups
- PD-L1 expression, somatic tumor mutations, and gene expression signatures should not be used in clinical practice to exclude patients from IO + TKI combination therapies
Dr. Xu then discussed biomarkers in high risk adjuvant therapy for RCC. The key question here is: Can we identify subgroups who may benefit from adjuvant PD-L1 (atezolizumab)? At the time of diagnosis higher kidney injury molecule-1 is associated with higher risk RCC, kidney injury molecule-1 differentiates benign versus malignant renal masses, and pre-nephrectomy kidney injury molecule-1 is associated with metastasis free and overall survival. In the adjuvant setting, in the ASSURE trial of adjuvant sunitinib, sorafenib, or placebo, higher levels of kidney injury molecule-1 in post-nephrectomy, pre-treatment plasma samples were associated with worse disease free and overall survival. Kidney injury molecule-1 can be measured in plasma or serum and is stable under different storage conditions, suggesting suitability to serve as a peripheral blood circulating marker. In the IMmotion010 analysis, kidney injury molecule-1 is both prognostic and predictive. Kidney injury molecule-1–high status was associated with reduced disease-free survival, and patients with kidney injury molecule-1 high had better disease-free survival with atezolizumab versus placebo (HR 1.75, 95% CI 1.40-2.17):
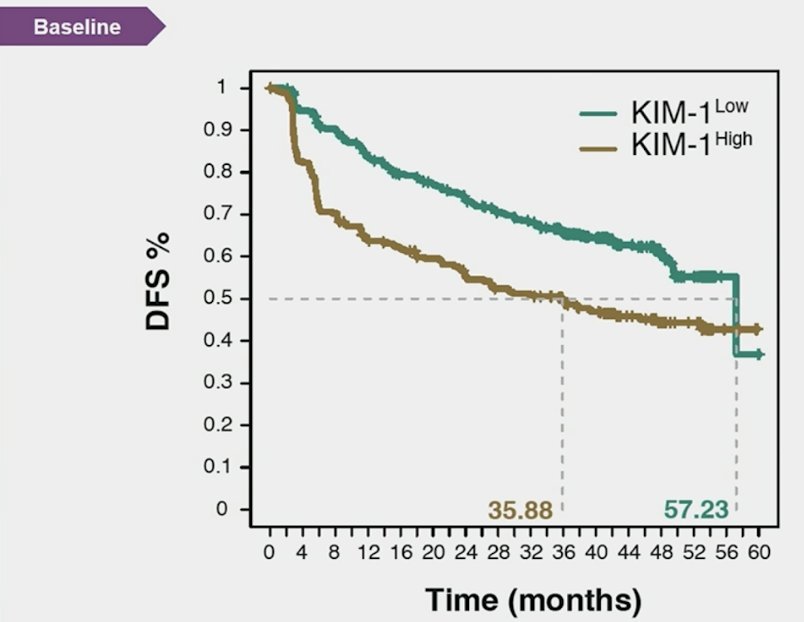
Atezolizumab improved disease free survival versus placebo in the baseline kidney injury molecule-1 high subgroup (HR 0.72, 95% CI 0.52-0.99), but not in the kidney injury molecule-1 low group:

Dr. Xu notes that reversal of the hazard ratios suggests an interaction between kidney injury molecule-1 high subgroup and atezolizumab effect on disease free survival. Of note, PD-L1 and T-effector high were not predictive of benefit from atezolizumab.
Dr. Xu emphasized that different kidney injury molecule-1 assays have high concordance relative to kidney injury molecule-1 measurements, but absolute values depend on the calibration and assay used. Importantly, cutoffs in the adjuvant setting are assay specific, and future prospective studies should standardize assay calibration to produce generalizable results. Importantly, there are several unanswered questions for adjuvant kidney injury molecule-1:
- What happens if patients have higher kidney injury molecule-1 immediately after partial nephrectomy?
- Controlling for surgery types (partial versus radical nephrectomy) may further strengthen the prognostic and predictive value of kidney injury molecule-1
- Does kidney injury molecule-1 help to select patients for adjuvant pembrolizumab? Are kidney injury molecule-1-low patients being overtreated with pembrolizumab and are kidney injury molecule-1-high patients being undertreated with pembrolizumab
- Is kidney injury molecule-1 additive with other potential adjuvant biomarkers?

Dr. Xu provided several concluding statements regarding kidney injury molecule-1 in IMmotion010:
- This analysis validates serum kidney injury molecule-1 as a prognostic and predictive biomarker for adjuvant immunotherapy
- Standardization of kidney injury molecule-1 assays and cutoffs are needed
- Are we ready to prospectively validate kidney injury molecule-1in adjuvant RCC trials? Yes we are
Presented by: Wenxin Xu, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024


